Abstract
Connexin (Cx) 43 is the predominant protein forming gap junctions and non‐junctional hemichannels in ventricular myocardium. The Cx43 proteins are central to the cardioprotection afforded by ischaemic preconditioning (IP). The specific role of mitochondrial C×43 in protection by IP is reviewed.
In the present review, the structure and regulation of connexin (Cx) 43‐formed hemichannels and gap junctions and their role in cardioprotection by ischaemic preconditioning (IP) are discussed. Furthermore, the presence of Cx43 at other subcellular locations within cardiomyocytes—particularly in the mitochondria—is considered in the context of IP.
Structure, function and regulation of connexins
The proteins of the connexin gene family, comprising 20 members in the mouse and 21 members in the human genome,1 are named according to their molecular weights. Most cells or tissues express more than one connexin—for example, Cx40, Cx43 and Cx45 in the adult myocardium. Cx40 is expressed mainly in the atria and Cx45 in the conduction system, whereas Cx43 is the predominant connexin in the ventricles (for review see van Veen et al2).
Cx43, like all other connexins, contains four transmembrane domains, two extracellular and one intracellular loop, and the amino and carboxy termini, which are located in the cytosol. The connexins differ in the length of their cytosolic carboxy terminus, which is characterised by the presence of several phosphorylation sites (except for Cx26, which is unphosphorylated3).
Cx43 is predominantly located in the sarcolemma, where six connexins assemble into a so‐called connexon or hemichannel. Two opposing connexons, one from each adjacent cell, form a pore, which is central to electrical cell coupling. Clusters of such pores form a gap junction. Gap junctions are not selective for specific ions4 and are permeable for molecules with a molecular weight up to 1000 Da. The flux of molecules through gap junctions is determined by the chemical and electrical gradient between two connected cells. Furthermore, the transfer of molecules between connected cells is regulated by the assembly and degradation of gap junctions,5 as well as by their open probability.6 Most of the ventricular Cx43‐formed connexons are located in the terminal intercalated disks and some in the lateral sides of cardiomyocytes.
Non‐junctional hemichannels have been shown to contribute to volume regulation,7 the release of ATP and NAD+ from the cytosol,8 and the activation of cell survival pathways.9 Under resting conditions, hemichannels are predominantly in a closed state and their gating is regulated, among other factors, by the phosphorylation status of Cx43 (for review see Saez et al10). The number of putative phosphorylation sites of Cx43 is species dependent: 24 phosphorylation sites in mice and 19 in humans are predicted.2 Cx43 is a target protein of several kinases, among them protein kinase A, protein kinase C (PKC), protein kinase G, protein tyrosine kinases, mitogen‐activated protein kinases and casein kinase, but also of protein phosphatases (for review see Schulz and Heusch11 and Lampe and Lau12). The phosphorylation of Cx43 at Ser368 by PKC induces hemichannels to close, whereas inhibition of PKC induces them to open.13,14
Analysis of protein–protein interactions shows that Cx43 is associated not only with protein kinases but also with a variety of other proteins. Among these interaction partners are adherens junction‐ and tight junction‐associated proteins such as β‐catenin or ZO‐1 and ZO‐2, cytoskeletal proteins such as actin and tubulin, and caveolin (for review see Wei et al15 and Giepmans16). Recently, connexin‐interacting protein 150—a protein with no conserved domains —was found to interact with Cx43,17 and connexin‐interacting protein 150 has been suggested to be involved in regulating the Cx43 content in the plasma membrane. The finding that Cx43 interacts with several proteins exerting different functions supports the idea that Cx43 not only acts as a channel‐forming protein, but is also involved in intracellular signalling.
Cx43 in ischaemia and IP
Under physiological conditions Cx43 is partially phosphorylated and remains phosphorylated during the first few minutes of ischaemia.18 Ischaemia affects the association of Cx43 with kinases such as c‐Src in astrocytes.19 Several studies have shown dephosphorylation of Cx43 with an increasing duration of myocardial ischaemia,18,20,21,22 a process associated with the opening of Cx43‐formed hemichannels.23,24 Ischaemia also induces translocation of Cx43 from the plasma membrane to an intracellular pool.20
IP, the reduction of infarct size by brief episodes of ischaemia and reperfusion preceding a period of sustained ischaemia/reperfusion, is known to affect the phosphorylation status of Cx43. In pig,18 rabbit,22 and rat hearts,21 IP attenuated the ischaemia‐induced dephosphorylation of Cx43 and the resulting electrical uncoupling.21 The preserved phosphorylation of Cx43 after IP not only may be caused by an enhanced association of Cx43 with kinases such as PKC and p3818 but may also be due to reduced co‐localisation with protein phosphatases.25
A so‐called death factor may spread from cell to cell through Cx43‐formed gap junctions at the time of reperfusion after sustained ischaemia.26,27 Uncoupling of gap junctions by heptanol may limit the spread of this factor—potentially sodium—and therefore the spatial progression of cell death.28,29,30,31 On the other hand, pretreatment with heptanol, which dissolves Cx43 from membranes, abolishes IP cardioprotection,32 suggesting that Cx43 is central to the signal transduction cascade of IP.
Further evidence for the involvement of Cx43 in IP cardioprotection comes from experiments on heterozygous Cx43‐deficient mice, where IP cardioprotection is lost.33,34 Cx43‐formed gap junctions are not required for IP protection, as loss of IP protection after simulated ischaemia/reperfusion is also seen in isolated cardiomyocytes of Cx43‐deficient mice.35 These findings imply a role of Cx43 in preconditioning that is independent of cell to cell communication.
Mitochondrial Cx43 in cardioprotection
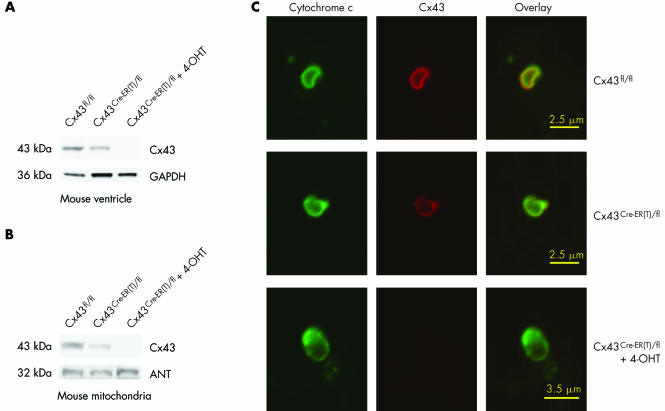
Although Cx43 is predominantly located in the sarcolemma, the protein is also present in other subcellular structures. The cytosolic carboxy terminus of rat Cx43 (residues 243–382) is associated with the nucleus of cardiomyocytes and HeLa cells, where the stable expression of carboxy terminal Cx43 inhibits cell proliferation.36 Furthermore, Cx43 is located in mitochondria of human umbilical vein endothelial cells, and the mitochondrial Cx43 content is increased in response to homocysteine‐induced cellular stress.37 We have recently presented evidence for the presence of Cx43 in mitochondria from mouse, rat, pig and human left ventricular myocardium obtained from fluorescence‐activated cell sorting and western blot analyses, as well as confocal and immunoelectron microscopy.38 In conditional Cx43 knockout mice the Cx43 content is reduced both in total ventricular extracts and in isolated mitochondria (fig 1). The location of Cx43 in mitochondria is of particular interest, because mitochondria are important signalling elements of IP.39,40,41,42 The mitochondrial content of Cx43 is increased very rapidly by IP (two 5 min cycles of ischaemia and reperfusion) and the enhanced Cx43 protein level in the mitochondria is maintained for at least 90 min in a pig model of IP.38 The subcellular origin of Cx43, which is targeted for mitochondrial import, has not been elucidated. However, the fact that the Cx43 content of the intercalated disks is unaffected by IP18 makes it unlikely that Cx43 is transported from the sarcolemma to the mitochondria. It is also unclear whether Cx43 forms pore structures in the mitochondrial membrane as it does in the sarcolemma.
Figure 1 (A) Western blot analysis for connexin (Cx) 43 or as loading control for GAPDH on 30 μg ventricular protein extracts of Cx43fl/fl, untreated Cx43Cre‐ER(T)/fl or 4‐hydroxytamoxifen (4‐OHT) ‐treated Cx43Cre‐ER(T)/fl mice. (B) Western blot analysis for Cx43 or for the mitochondrial marker protein ANT on 30 μg protein extracts of ventricular mitochondria isolated from Cx43fl/fl, untreated Cx43Cre‐ER(T)/fl or 4‐OHT‐treated Cx43Cre‐ER(T)/fl mice. (C) Mitochondria isolated from the ventricles of Cx43fl/fl, untreated Cx43Cre‐ER(T)/fl or 4‐OHT‐treated Cx43Cre‐ER(T)/fl mice were stained with antibodies against Cx43 (red) or the mitochondrial marker cytochrome c (green) and analysed by confocal laser scan microscopy. Merged image shows co‐localisation pixels in yellow. Reproduced with permission from Boengler et al.38
Co‐immunoprecipitation studies have shown an interaction of Cx43 with Tom20, which is, with Tom5, 6, 7, 22, 40 and 70, part of the translocase of the outer membrane (TOM) protein complex and thereby of the general mitochondrial import machinery. Ischaemia decreases the mitochondrial protein level of Tom20, which functions as a presequence receptor for proteins to be imported into the mitochondria. However, in mitochondria of preconditioned pig myocardium, the Tom20 protein level is preserved.43 The sustained Tom20 protein level may reflect overall preservation of the TOM complex during ischaemia after IP. Preservation of the TOM complex may enhance the translocation of Cx43 to cardiomyocyte mitochondria and thereby contribute to the increased mitochondrial Cx43 protein level after IP.
Functional role of mitochondrial Cx43
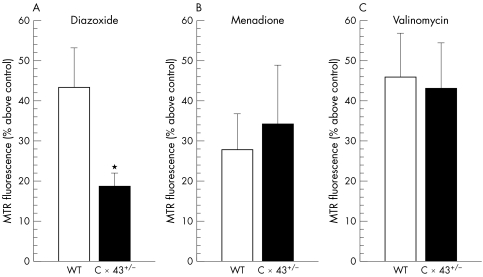
Reactive oxygen species (ROS) are produced in part by uncoupling of the oxidative phosphorylation during ischaemia/reperfusion and contribute to cardiomyocyte damage (for review see Ferrari44). Whereas excessive ROS formation is detrimental to cardiomyocytes, small amounts of ROS trigger IP cardioprotection. ROS are a central step in the IP signal transduction cascade leading to activation of PKC45 or to phosphorylation of p38 mitogen‐activated protein kinase and nuclear translocation of nuclear factor κB.46 ROS are also second messengers in pharmacological preconditioning with diazoxide.47,48 Accordingly, we recently showed that diazoxide impaired ROS generation in heterozygous Cx43‐deficient cardiomyocytes compared with wild‐type cardiomyocytes. Loss of ROS formation in Cx43‐deficient cardiomyocytes was specific for diazoxide, as ROS formation did not change in response to the potassium ionophore valinomycin and to menadione, which non‐specifically induces ROS formation (fig 2). Consequently, diazoxide reduced infarct size in wild‐type but not in heterozygous Cx43‐deficient mice. In contrast, menadione reduced infarct size regardless of the genotype.49 Therefore, heterozygous Cx43‐deficient cardiomyocytes have a specific functional deficit in ROS formation in response to diazoxide and accordingly have less protection. However, the function of Cx43 in mitochondria and its role in IP cardioprotection remains to be elucidated in more detail.
Figure 2 (A) Increase in MitotrackerRed (MTR) fluorescence reflecting reactive oxygen species (ROS) formation in response to diazoxide (200 µmol/l) above vehicle control was attenuated in cardiomyocytes from connexin (Cx) 43+/– (n = 10 mice) v wild type (WT) (n = 12 mice). (B) ROS formation triggered by menadione (2 µmol/l) was not different between Cx43+/– (n = 5 mice) and WT (n = 5 mice). (C) ROS formation by valinomycin (10 nmol/l) was also not different between Cx43+/– (n = 5 mice) and WT (n = 5 mice). *p < 0.05 v WT. Reproduced with permission from Heinzel et al.49
Conclusion
In all species analysed so far IP has been shown to be cardioprotective.50 There is now evidence that IP‐induced and diazoxide‐induced protection depend on Cx43 but not on the presence of gap junctions. Instead, the location of Cx43 in mitochondria, which are organelles central to IP signal transduction, and the involvement of ROS formation suggest that Cx43 has a role in IP cardioprotection beyond its channel‐forming properties in the sarcolemma.
ACKNOWLEDGEMENTS
The authors' studies were supported by the German Research Foundation (Schu843/7‐1) and the ISHR Research Fellowship to KB.
Abbreviations
Cx - connexin
IP - ischaemic preconditioning
PKC - protein kinase C
ROS - reactive oxygen species
TOM - translocase of the outer membrane
References
- 1.Söhl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 200462228–232. [DOI] [PubMed] [Google Scholar]
- 2.Van Veen T A B, van Rijen H V M, Opthof T. Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc Res 200151217–229. [DOI] [PubMed] [Google Scholar]
- 3.Seaz J C, Nairn A C, Czernik A J.et al Phosphorylation of connexin 32, a hepatocyte gap junction protein, by cAMP‐dependet protein kinase, protein kinase C and Ca2+/calmodulin‐dependent protein kinase II. Eur J Biochem 1990192263–273. [DOI] [PubMed] [Google Scholar]
- 4.Veenstra R D, Wang H ‐ Z, Beblo D A.et al Selectivity of connexin‐specific gap junctions does not correlate with channel conductance. Circ Res 1995771156–1165. [DOI] [PubMed] [Google Scholar]
- 5.Saez J C, Berthoud V M, Branes M C.et al Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 2003831359–1400. [DOI] [PubMed] [Google Scholar]
- 6.Spray D C, Burt J M. Structure‐activity relations of the cardiac gap junction channel. Am J Physiol Cell Physiol 1990258C195–C205. [DOI] [PubMed] [Google Scholar]
- 7.Quist A P, Rhee S K, Lin H.et al Physiological role of gap‐junctional hemichannels: extracellular calcium‐dependent isosmotic volume regulation. J Cell Biol 20001481063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Maio A, Vega V L, Contreras J E. Gap junctions, homeostasis, and injury. J Cell Physiol 2002191269–282. [DOI] [PubMed] [Google Scholar]
- 9.Plotkin L I, Bellido T. Bisphosphonate‐induced, hemichannel‐mediated, anti‐apoptosis through the Src/ERK pathway: a gap junction‐independent action of connexin43. Cell Commun Adhes 20018377–382. [DOI] [PubMed] [Google Scholar]
- 10.Saez J C, Retamal M A, Basilio D.et al Connexin‐based gap junction hemichannels: gating mechanisms. Biochem Biophys Acta 20051711215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz R, Heusch G. Connexin 43 and ischemic preconditioning. Cardiovasc Res 200462335–344. [DOI] [PubMed] [Google Scholar]
- 12.Lampe P D, Lau A F. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys 2000384205–215. [DOI] [PubMed] [Google Scholar]
- 13.Bao X, Altenberg G A, Reuss L. Mechanisms of regulation of the gap junction protein connexin 43 by protein kinase C‐mediated phosphorylation. Am J Physiol Cell Physiol 2004286C647–C654. [DOI] [PubMed] [Google Scholar]
- 14.Bao X, Reuss L, Altenberg G A. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C‐mediated phosphorylation of serine 368. J Biol Chem 200427920058–20066. [DOI] [PubMed] [Google Scholar]
- 15.Wei C ‐ J, Xu X, Lo C W. Connexins and cell signalling in development and disease. Annu Rev Cell Dev Biol 200420811–838. [DOI] [PubMed] [Google Scholar]
- 16.Giepmans B N G. Gap junctions and connexin‐interacting proteins. Cardiovasc Res 200462233–245. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama M, Ishida N, Ogawa T.et al Molecular cloning and functional analysis of a novel Cx43 partner protein CIP150. Biochem Biophys Res Commun 20053351264–1271. [DOI] [PubMed] [Google Scholar]
- 18.Schulz R, Gres P, Skyschally A.et al Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J 2003171355–1357. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Hertzberg E L, Spray D C. Regulation of connexin 43‐protein binding in astrocytes in response to chemical ischemia/hypoxia. J Biol Chem 20052807941–7948. [DOI] [PubMed] [Google Scholar]
- 20.Beardslee M A, Lerner D L, Tadros P N.et al Dephosphorylation and intracellular redistribution of ventricular connexin 43 during electrical uncoupling induced by ischemia. Circ Res 200087656–662. [DOI] [PubMed] [Google Scholar]
- 21.Jain S K, Schuessler R B, Saffitz J E. Mechanisms of delayed electrical uncoupling induced by ischemic preconditioning. Circ Res 2003921138–1144. [DOI] [PubMed] [Google Scholar]
- 22.Miura T, Ohnuma Y, Kuno A.et al Protective role of gap junctions in preconditioning against myocardial infarction. Am J Physiol Heart Circ Physiol 2004286H214–H221. [DOI] [PubMed] [Google Scholar]
- 23.John S A, Kondo R, Wang S ‐ Y.et al Connexin‐43 hemichannels opened by metabolic inhibition. J Biol Chem 1999274236–240. [DOI] [PubMed] [Google Scholar]
- 24.Li F, Sugishita K, Su Z.et al Activation of connexin‐43 hemichannels can elevate [Ca2+]i and [Na+]i in rabbit ventricular myocytes during metabolic inhibition. J Mol Cell Cardiol 2001332145–2155. [DOI] [PubMed] [Google Scholar]
- 25.Konietzka I, Gres P, Heusch G.et al Co‐localization of connexin 43 (Cx43) and protein phosphatases in preconditioned myocardium in pigs [abstract]. J Mol Cell Cardiol 200436757 [Google Scholar]
- 26.Azzam E I, de Toledo S M, Little J B. Direct evidence for the participation of gap junction‐mediated intercellular communication in the transmission of damage signals from alpha‐particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA 200198473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia‐Dorado D, Ruiz‐Meana M, Padilla F.et al Gap‐junction‐mediated intercellular communication in ischemic preconditioning. Cardiovasc Res 200255456–465. [DOI] [PubMed] [Google Scholar]
- 28.Garcia‐Dorado D, Rodriguez‐Sinovas A, Ruiz‐Maena M. Gap junction‐mediated spread of cell injury and death during myocardial ischemia‐reperfusion. Cardiovasc Res 200461386–401. [DOI] [PubMed] [Google Scholar]
- 29.Garcia‐Dorado D, Inserte J, Ruiz‐Meana M.et al Gap junction uncoupler heptanol prevents cell‐to‐cell progression of hypercontracture and limits necrosis during myocardial reperfusion. Circulation 1997963579–3586. [DOI] [PubMed] [Google Scholar]
- 30.Chen B ‐ P, Mao H ‐ J, Fan F ‐ Y.et al Delayed uncoupling contributes to the protective effect of heptanol against ischaemia in the rat isolated heart. Clin Exp Pharmacol Physiol 200532655–662. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez‐Sinovas A, Garcia‐Dorado D, Ruiz‐Meana M.et al Protective effect of gap junction uncouplers given during hypoxia against reoxygenation injury in isolated rat hearts. Am J Physiol Heart Circ Physiol 2006290H648–H656. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Whittaker P, Yao M.et al The gap junction uncoupler heptanol abrogates infarct size reduction with preconditioning in mouse hearts. Cardiovasc Pathol 200211158–165. [DOI] [PubMed] [Google Scholar]
- 33.Schwanke U, Konietzka I, Duschin A.et al No ischemic preconditioning in heterozygous connexin43‐deficient mice. Am J Physiol Heart Circ Physiol 2002283H1740–H1742. [DOI] [PubMed] [Google Scholar]
- 34.Schwanke U, Li X, Schulz R.et al No ischemic preconditioning in heterozygous connexin 43‐deficient mice: a further in vivo study. Basic Res Cardiol 200398181–182. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Heinzel F R, Boengler K.et al Role of connexin 43 in ischemic preconditioning does not involve intercellular communications through gap junctions. J Mol Cell Cardiol 200436161–163. [DOI] [PubMed] [Google Scholar]
- 36.Dang X, Doble B W, Kardami E. The carboxy‐tail of connexin‐43 localizes to the nucleus and inhibits cell growth. Mol Cell Biochem 200324235–38. [PubMed] [Google Scholar]
- 37.Li H, Brodsky S, Kumari S.et al Paradoxical overexpression and translocation of connexin 43 in homocysteine‐treated endothelial cells. Am J Physiol Heart Circ Physiol 2001282H2124–H2133. [DOI] [PubMed] [Google Scholar]
- 38.Boengler K, Dodoni G, Ruiz‐Meana M.et al Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res 200567234–244. [DOI] [PubMed] [Google Scholar]
- 39.O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res 200494420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halestrap A P, Clarke S J, Javadov S A. Mitochondrial permeability transition pore opening during myocardial reperfusion: a target for cardioprotection. Cardiovasc Res 200461372–385. [DOI] [PubMed] [Google Scholar]
- 41.Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res 2004947–16. [DOI] [PubMed] [Google Scholar]
- 42.Krieg T, Cohen M V, Downey J M. Mitochondria and their role in preconditioning's trigger phase. Basic Res Cardiol 200398228–234. [DOI] [PubMed] [Google Scholar]
- 43.Boengler K, Dodoni G, Gres P.et al Connexin 43 is located at the inner membrane of cardiomyocyte mitochondria and interacts with the translocase of the outer membrane (TOM) [abstract]. Circulation 2005112157 [Google Scholar]
- 44.Ferrari R. The role of mitochondria in ischemic heart disease. J Cardiovasc Pharmacol 199628(Suppl 1)S1–10. [DOI] [PubMed] [Google Scholar]
- 45.Baines C P, Goto M, Downey J M. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol 199729207–216. [DOI] [PubMed] [Google Scholar]
- 46.Das D K, Maulik N, Sato M.et al Reactive oxygen species function as second messenger during ischemic preconditioning of heart. Mol Cell Biochem 199919659–67. [PubMed] [Google Scholar]
- 47.Pain T, Yang X ‐ M, Critz S D.et al Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res 200087460–466. [DOI] [PubMed] [Google Scholar]
- 48.Forbes R A, Steenbergen C, Murphy E. Diazoxide‐induced cardioprotection requires signaling through a redox‐sensitive mechanism. Circ Res 200188802–809. [DOI] [PubMed] [Google Scholar]
- 49.Heinzel F R, Luo Y, Li X.et al Impairment of diazoxide‐induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res 200597583–586. [DOI] [PubMed] [Google Scholar]
- 50.Heusch G. Nitroglycerin and delayed preconditioning in humans. yet another new mechanism for an old drug ? Circulation 20011032876–2878. [DOI] [PubMed] [Google Scholar]