Abstract
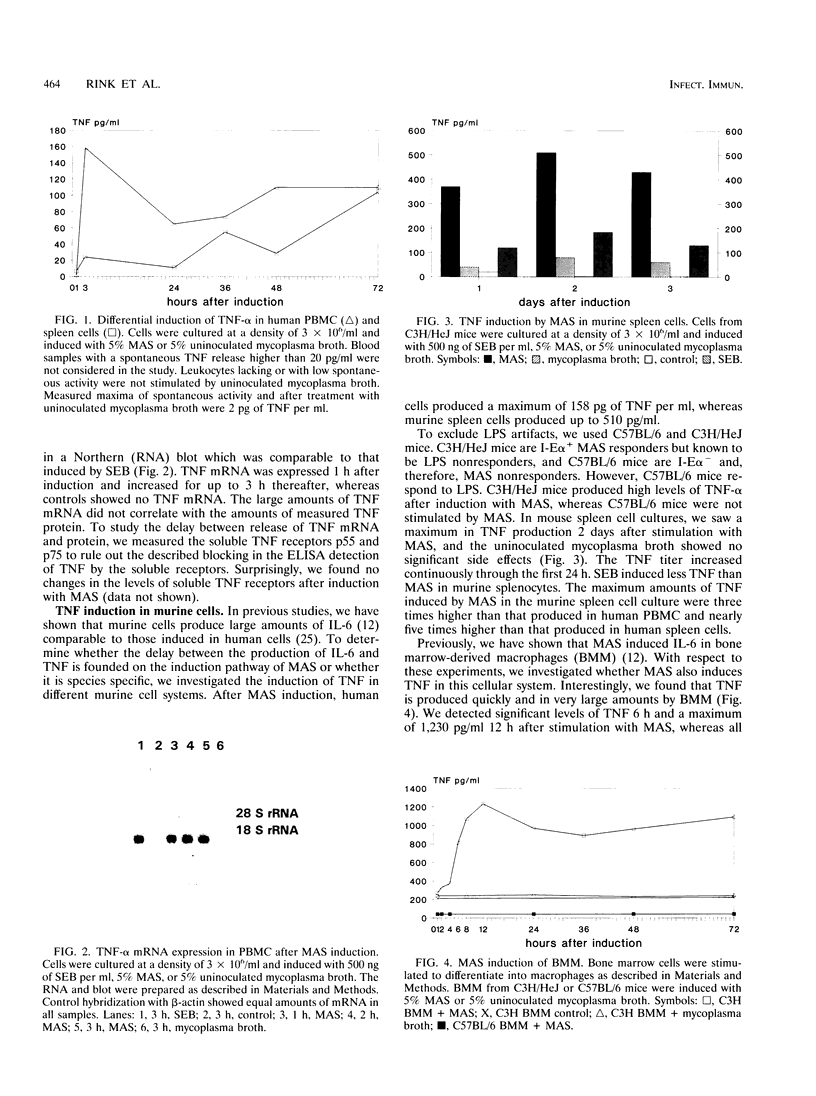
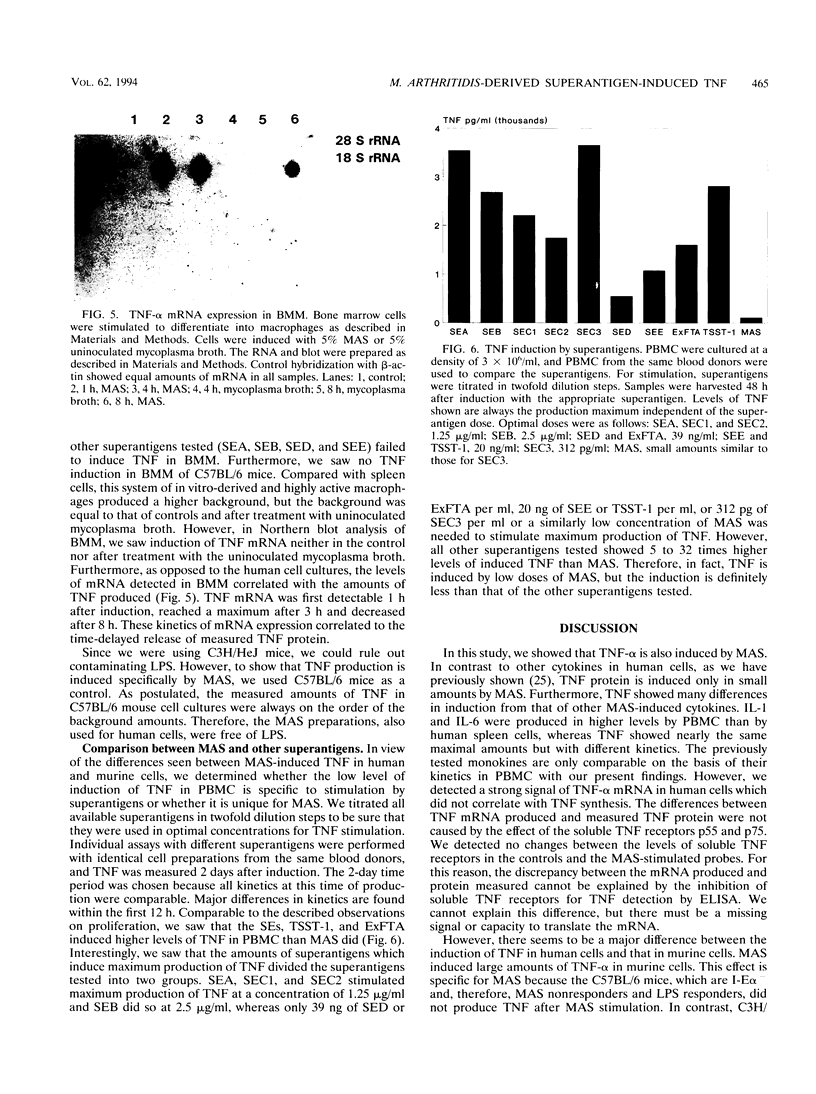
Mycoplasma arthritidis-derived superantigen (MAS) is exclusively produced by M. arthritidis, which is the only known mycoplasma to produce a superantigen. As a superantigen, MAS shows properties similar to those of the staphylococcal enterotoxins and related substances, such as binding to major histocompatibility complex (MHC) class II and V beta-specific stimulation of T cells. In this series of experiments, we demonstrate some differences between MAS and other superantigens. MAS induced the production of tumor necrosis factor alpha (TNF-alpha) mRNA in human as well as in murine leukocytes. However, only in murine leukocytes was the mRNA adequately translated into the protein. In human peripheral blood mononuclear cells, we found only small amounts of TNF, whereas in murine spleen cells we detected levels more than three times higher. The proliferative response to MAS has been shown to be restricted to I-E alpha in the murine MHC. Furthermore, TNF was induced in I-E alpha+ bone marrow-derived macrophages by MAS. In these cells, MAS rapidly induced very high levels of TNF and the amounts of mRNA detected correlated to the amount of protein produced. In comparison with other superantigens, including the staphylococcal enterotoxins, toxic shock syndrome toxin 1, and exfoliative toxin A, the failure of MAS to induce TNF-alpha in human peripheral blood mononuclear cells is specific for MAS and not common to all superantigens. The direct activation of bone marrow-derived macrophages also seems to be specific for MAS. These data suggest that the induction of TNF-alpha by MAS is dependent on the strength of binding to the MHC class II molecule.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccala R., Smith L. R., Vestberg M., Peterson P. A., Cole B. C., Theofilopoulos A. N. Mycoplasma arthritidis mitogen. V beta engaged in mice, rats, and humans, and requirement of HLA-DR alpha for presentation. Arthritis Rheum. 1992 Apr;35(4):434–442. doi: 10.1002/art.1780350413. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N., Santhanam U., Lau L. L., Tatter S. B., Ghrayeb J., Rivelis M., Steinman R. M., Sehgal P. B., May L. T. IL-6/IFN-beta 2 in synovial effusions of patients with rheumatoid arthritis and other arthritides. Identification of several isoforms and studies of cellular sources. J Immunol. 1989 Oct 1;143(7):2153–2159. [PubMed] [Google Scholar]
- Cannon G. W., Cole B. C., Ward J. R., Smith J. L., Eichwald E. J. Arthritogenic effects of Mycoplasma arthritidis T cell mitogen in rats. J Rheumatol. 1988;15(5):735–741. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. I. Transformation is associated with an H-2-linked gene that maps to the I-E/I-C subregion. J Immunol. 1981 Nov;127(5):1931–1936. [PubMed] [Google Scholar]
- Cole B. C., Piepkorn M. W., Wright E. C. Influence of genes of the major histocompatibility complex on ulcerative dermal necrosis induced in mice by Mycoplasma arthritidis. J Invest Dermatol. 1985 Oct;85(4):357–361. doi: 10.1111/1523-1747.ep12276973. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N., Hassell L. A., Washburn L. R., Ward J. R. Toxicity but not arthritogenicity of Mycoplasma arthritidis for mice associates with the haplotype expressed at the major histocompatibility complex. Infect Immun. 1983 Sep;41(3):1010–1015. doi: 10.1128/iai.41.3.1010-1015.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz J. N., Cole B. C. Direct activation of the J774.1 Murine macrophage cell line by mycoplasma arthritidis. Infect Immun. 1982 Aug;37(2):811–819. doi: 10.1128/iai.37.2.811-819.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Hartwig U. T-lymphocyte stimulation by microbial superantigens. Chem Immunol. 1992;55:36–64. [PubMed] [Google Scholar]
- Fleischer B., Mittrücker H. W., Metzroth B., Braun M., Hartwig U. Mitogenic toxins as MHC class II-dependent probes for T cell antigen receptors. Behring Inst Mitt. 1991 Feb;(88):170–176. [PubMed] [Google Scholar]
- Homfeld J., Homfeld A., Nicklas W., Rink L., Weyland A., Kirchner H. Induction of interleukin-6 in murine bone marrow-derived macrophages stimulated by the Mycoplasma arthritidis mitogen MAS. Autoimmunity. 1990;7(4):317–327. doi: 10.3109/08916939009087591. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson E., Miettinen A., Hakkarainen K., Backman A., Seniusová B., Mäkisara P., Rokkanen P. Cultivation of fastidious mycoplasmas from human arthritis. Z Rheumatol. 1983 Mar-Apr;42(2):66–69. [PubMed] [Google Scholar]
- Jupin C., Anderson S., Damais C., Alouf J. E., Parant M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and gamma interferon. J Exp Med. 1988 Mar 1;167(3):752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Bauer A., Moritz T., Herbst F. Lymphocyte activation and induction of interferon gamma in human leucocyte cultures by the mitogen in Mycoplasma arthritidis supernatant (MAS). Scand J Immunol. 1986 Nov;24(5):609–613. doi: 10.1111/j.1365-3083.1986.tb02177.x. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Brehm G., Nicklas W., Beck R., Herbst F. Biochemical characterization of the T-cell mitogen derived from Mycoplasma arthritidis. Scand J Immunol. 1986 Sep;24(3):245–249. doi: 10.1111/j.1365-3083.1986.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Nicklas W., Giebler D., Keyssner K., Berger R., Storch E. Induction of interferon gamma in mouse spleen cells by culture supernatants of mycoplasma arthritidis. J Interferon Res. 1984 Summer;4(3):389–397. doi: 10.1089/jir.1984.4.389. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Wright S. C. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J. 1990 Nov;4(14):3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Cell biology of the mycoplasmas. Bacteriol Rev. 1972 Sep;36(3):263–290. doi: 10.1128/br.36.3.263-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Matthes M., Schrezenmeier H., Homfeld J., Fleischer S., Malissen B., Kirchner H., Fleischer B. Clonal analysis of human T cell activation by the Mycoplasma arthritidis mitogen (MAS). Eur J Immunol. 1988 Nov;18(11):1733–1737. doi: 10.1002/eji.1830181112. [DOI] [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink L., Kirchner H. Mycoplasma arthritidis-derived superantigen. Chem Immunol. 1992;55:137–145. [PubMed] [Google Scholar]
- Rink L., Kruse A., Nicklas W., Hoyer J., Kirchner H. Induction of cytokines in human peripheral blood and spleen cells by the Mycoplasma arthritidis-derived superantigen. Lymphokine Cytokine Res. 1992 Apr;11(2):105–108. [PubMed] [Google Scholar]
- Ruuth E., Praz F. Interactions between mycoplasmas and the immune system. Immunol Rev. 1989 Dec;112:133–160. doi: 10.1111/j.1600-065x.1989.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Schober I., Braun R., Reiser H., Munk K., Leroux M., Kirchner H. la-positive T lymphocytes are the producer cells of interferon gamma. Exp Cell Res. 1984 Jun;152(2):348–356. doi: 10.1016/0014-4827(84)90636-0. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Woodward J. G. Yersinia enterocolitica produces superantigenic activity. J Immunol. 1992 Jan 1;148(1):225–233. [PubMed] [Google Scholar]
- Weisburg W. G., Tully J. G., Rose D. L., Petzel J. P., Oyaizu H., Yang D., Mandelco L., Sechrest J., Lawrence T. G., Van Etten J. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989 Dec;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]




