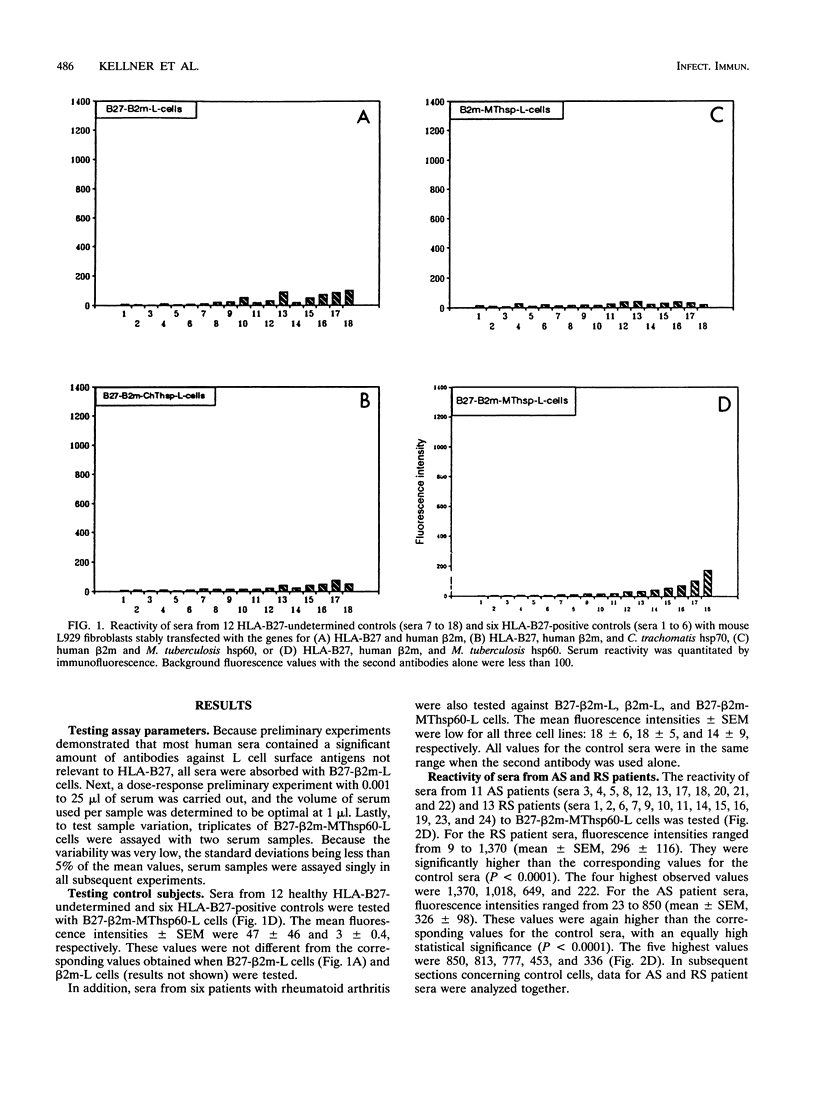
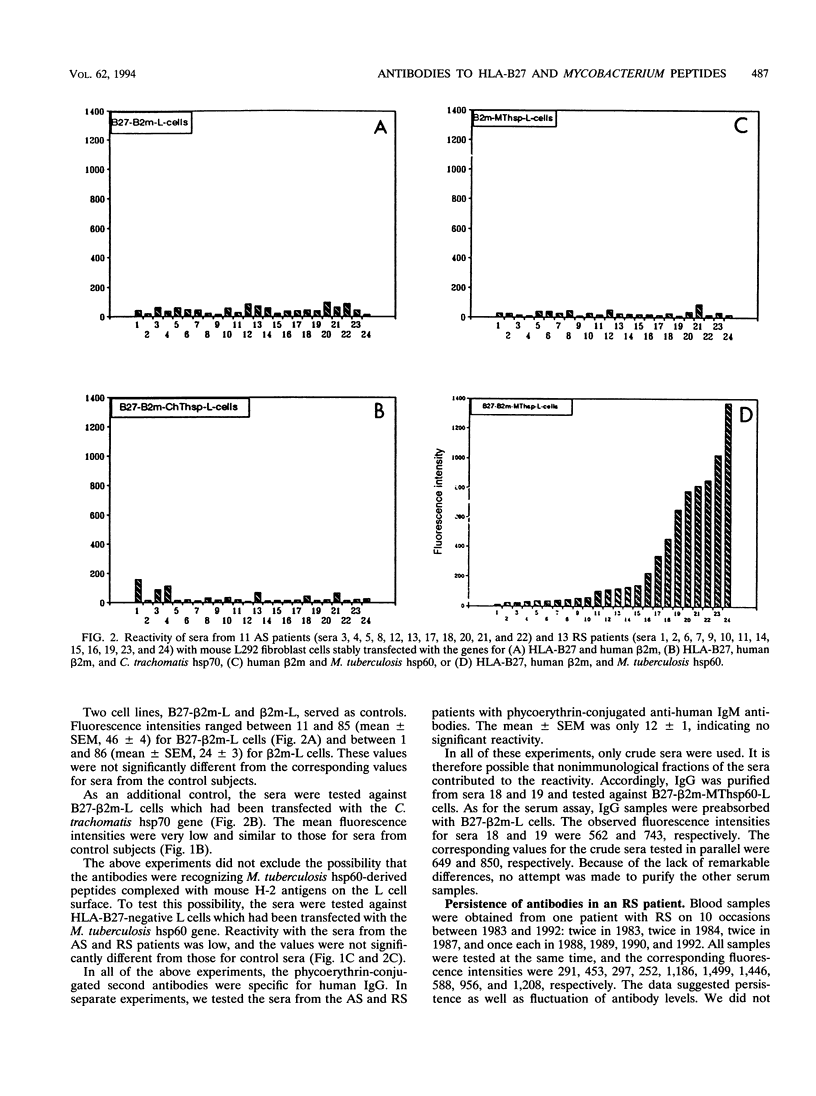
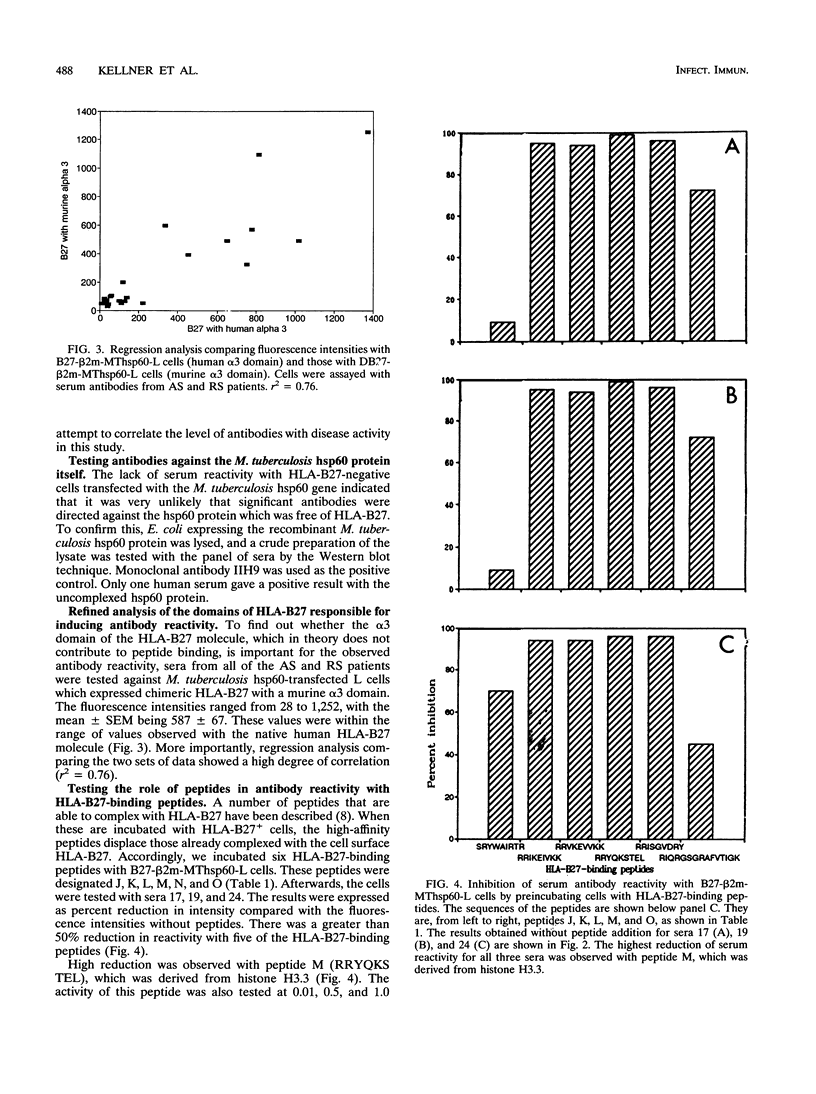
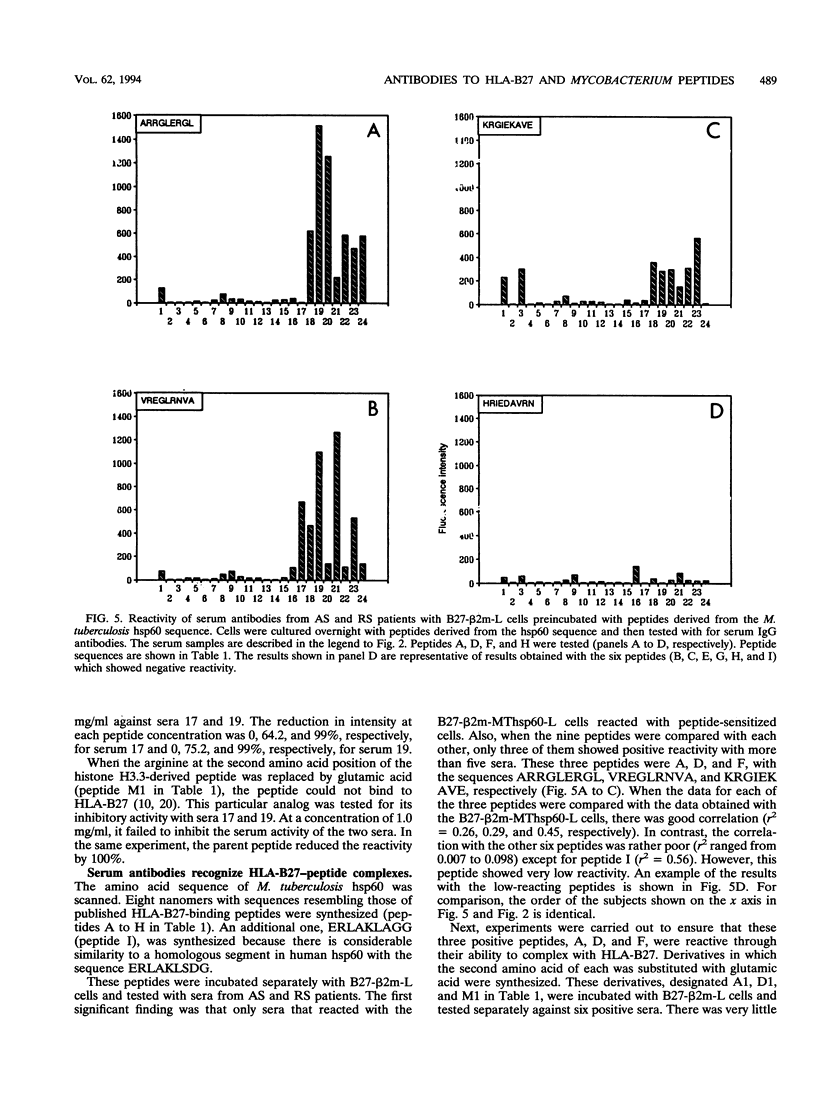
Abstract
HLA-B27-related arthritis is probably mediated by an immune response against HLA-B27 complexed with peptides derived from proteins of arthritis-causing bacteria. Immunogenic proteins with a high degree of homology among bacteria, such as in the hsp60 family, are likely candidates. To create such complexes experimentally, we transfected an HLA-B27 cell line with the Mycobacterium tuberculosis hsp60 gene. Because of previous observations that HLA-B27-peptide complexes can be distinguished by antibodies, we tested the transfected cell line with a panel of sera from 24 HLA-B27+ arthritis patients. Significant antibodies were detected in at least eight of the sera. Several cell lines and peptides were used as negative controls to ensure that the antibody reactivity was specific to HLA-B27-peptide complexes. A panel of nine peptides derived from the sequence of the Mycobacterium hsp60 were synthesized and tested. At least three were identified as being responsible for the serological activities.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Hämmerling G. J. MHC class-I transgenic mice. Annu Rev Immunol. 1991;9:297–322. doi: 10.1146/annurev.iy.09.040191.001501. [DOI] [PubMed] [Google Scholar]
- Benjamin R., Parham P. HLA-B27 and disease: a consequence of inadvertent antigen presentation? Rheum Dis Clin North Am. 1992 Feb;18(1):11–21. [PubMed] [Google Scholar]
- Bluestone J. A., Jameson S., Miller S., Dick R., 2nd Peptide-induced conformational changes in class I heavy chains alter major histocompatibility complex recognition. J Exp Med. 1992 Dec 1;176(6):1757–1761. doi: 10.1084/jem.176.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilition S. L., Maclean I. W., Peeling R., Winston S., Brunham R. C. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect Immun. 1990 Jan;58(1):189–196. doi: 10.1128/iai.58.1.189-196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Granfors K., Ogasawara M., Hill J. L., Lahesmaa-Rantala R., Toivanen A., Yu D. T. Analysis of IgA antibodies to lipopolysaccharide in Yersinia-triggered reactive arthritis. J Infect Dis. 1989 Jun;159(6):1142–1147. doi: 10.1093/infdis/159.6.1142. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Maika S. D., Richardson J. A., Tang J. P., Taurog J. D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990 Nov 30;63(5):1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Keat A., Thomas B., Dixey J., Osborn M., Sonnex C., Taylor-Robinson D. Chlamydia trachomatis and reactive arthritis: the missing link. Lancet. 1987 Jan 10;1(8524):72–74. doi: 10.1016/s0140-6736(87)91910-6. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The three-dimensional structure of HLA-B27 at 2.1 A resolution suggests a general mechanism for tight peptide binding to MHC. Cell. 1992 Sep 18;70(6):1035–1048. doi: 10.1016/0092-8674(92)90252-8. [DOI] [PubMed] [Google Scholar]
- McLain L., Dimmock N. J. A monoclonal antibody produced during infection which recognizes an epitope of influenza hemagglutinin only in the context of H-2k MHC class I antigen. J Immunol. 1993 Apr 15;150(8 Pt 1):3421–3426. [PubMed] [Google Scholar]
- Murphy D. B., Rath S., Pizzo E., Rudensky A. Y., George A., Larson J. K., Janeway C. A., Jr Monoclonal antibody detection of a major self peptide. MHC class II complex. J Immunol. 1992 Jun 1;148(11):3483–3491. [PubMed] [Google Scholar]
- Parham P. Purification of immunologically active HLA-A and -B antigens by a series of monoclonal antibody columns. J Biol Chem. 1979 Sep 25;254(18):8709–8712. [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Pope R. M., Lovis R. M., Gupta R. S. Activation of synovial fluid T lymphocytes by 60-kd heat-shock proteins in patients with inflammatory synovitis. Arthritis Rheum. 1992 Jan;35(1):43–48. doi: 10.1002/art.1780350107. [DOI] [PubMed] [Google Scholar]
- Schlosstein L., Terasaki P. I., Bluestone R., Pearson C. M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973 Apr 5;288(14):704–706. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- Schwimmbeck P. L., Yu D. T., Oldstone M. B. Autoantibodies to HLA B27 in the sera of HLA B27 patients with ankylosing spondylitis and Reiter's syndrome. Molecular mimicry with Klebsiella pneumoniae as potential mechanism of autoimmune disease. J Exp Med. 1987 Jul 1;166(1):173–181. doi: 10.1084/jem.166.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]



