Abstract
Isolated ventricular non‐compaction (IVNC) in adults is a genetic cardiac disease of emerging importance with a distinct clinical and pathophysiological presentation. The body of evidence for the underlying genetic basis of the disease has also grown. Prognosis remains poor for patients with impaired systolic left ventricular function, as treatment options are very limited. The diagnosis of IVNC, however, is often missed, most often as a consequence of ignorance of the condition. The relevant clinical issues and the emerging concepts of the aetiology of IVNC are summarised.
Isolated ventricular non‐compaction (IVNC) is a cardiomyopathy caused by intrauterine arrest of compaction of the myocardial fibres and meshwork, an important process in myocardial development.1 During intrauterine life, deep intertrabecular recesses communicating with the ventricular endocardium evolve. Subsequently, the myocardium condenses and the intertrabecular recesses are converted to capillaries.2 IVNC is characterised by the presence of deep intertrabecular recesses in hypertrophied and often hypokinetic segments of the myocardium of the left ventricle. By definition, IVNC occurs in the absence of other structural heart disease.3 Owing both to heterogeneity in its clinical presentation and to simple ignorance, IVNC may often be undiagnosed. The objective of this review, therefore, is to clarify all relevant clinical, diagnostic, pathoanatomical and genetic aspects of IVNC in adults and to discuss emerging concepts regarding this disease.
Previous clinical IVNC studies have been done on relatively small patient cohorts, and those studies were virtually all observational and retrospective in design. Prospective randomised clinical trials have not been conducted to date. The whole IVNC literature was previously based, to a considerable extent, on case reports sometimes of just one or a few patients.4,5,6,7,8 Most of the case reports were published during the past decade, reflecting the increasing awareness of this disease. However, this review is essentially based on available studies of larger patient series and with statistical analysis.
In addition, we use our own extensive experience with the diagnosis of the disease over 20 years to assess the literature critically.
HISTORY
The persistence of “sinusoids” in the left ventricle as an isolated anomaly was first described in the case of a 33‐year‐old woman in 1984, assessed by two‐dimensional echocardiography.9 One year later, our group reported the case of a 21‐year‐old female patient who first experienced an episode of acute left heart failure at the age of 15.10 Angiography showed a honeycomb‐like structure of the inner left ventricular wall. This initially suggested the suspicion of an atypical dilated cardiomyopathy. Two‐dimensional echocardiography showed channel‐like structures in a thickened myocardium of a hypokinetic left ventricle. One year later, another case of a 21‐year‐old male student with similar angiographic and echocardiographic findings was described.11 In that case, an autopsy proved for the first time the diagnosis of persistent sinusoids, today called IVNC, in a thickened myocardium. Four years later Chin et al12 presented a study of eight cases of IVNC in a paediatric population, and seven years after that we published a 10‐year experience with IVNC in adults, reporting 17 cases in 37 555 transthoracic echocardiographic studies.13
WORLD HEALTH ORGANIZATION CLASSIFICATION
The 1995 report of the World Health Organization and of the International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies defined cardiomyopathies as diseases of the myocardium associated with cardiac dysfunction. They distinguished five major disease entities (table 1).
Table 1 Classification of cardiomyopathies.
| 1. | Dilated cardiomyopathy |
| 2. | Hypertrophic cardiomyopathy |
| 3. | Restrictive cardiomyopathy |
| 4. | Arrhythmogenic right ventricular cardiomyopathy |
| 5. | Unclassified cardiomyopathies |
The category of unclassified cardiomyopathies is certainly unsatisfying, as it reflects our lack of understanding of diseases that are not yet fully accessible to current diagnostic tools. A variety of diseases such as fibroelastosis, amyloidosis and mitochondrial cardiomyopathies were therefore subsumed under unclassified cardiomyopathies. IVNC of the myocardium is, according to the WHO's classification, just another unclassified cardiomyopathy.14
EPIDEMIOLOGY
In a recent epidemiological study of primary cardiomyopathy in Australian children, IVNC accounted for 9.2% of all cases, identified as the third most frequent cardiomyopathy after dilated and hypertrophic cardiomyopathies.15 This is comparable with the experience at a single institution where IVNC was responsible for 9.5% of cardiomyopathies diagnosed in children over a five‐year period.16 In the largest series of adult patients with IVNC,17 the prevalence was 0.014% in patients referred to the echocardiography laboratory. However, this population consisted of patients referred to a University centre either because of uncertain echocardiographic findings or for further investigation of symptoms of severe heart failure. Because of the selection bias in a group of mainly symptomatic patients, the true prevalence of IVNC in adults remains unclear.
CLINICAL MANIFESTATIONS
The triad of heart failure symptoms, arrhythmias and embolic events is the major clinical manifestation in patients with reduced systolic left ventricular function13 and is comparable in adult and paediatric12 populations. Various patterns of arrhythmias, ranging from atrial fibrillation to sustained ventricular tachycardia, can be observed.
The diagnosis of IVNC was often delayed; in the cohort presented by Ritter et al13 most patients had been referred to the cardiology outpatients' clinic for evaluation of unexplained heart failure or inconclusive results on echocardiographic or angiographic studies performed elsewhere. In that study the mean (SD) time elapsed from onset of symptoms to correct diagnosis was 3.5 (5.7) years. Nine of 17 (53%) patients had clinical signs of heart failure, whereas eight patients were either asymptomatic or had uncharacteristic symptoms.
In a more recent publication, long‐term follow up of 34 adult patients was reported by Oechslin et al.17 This is the largest data series for adult patients. With this larger cohort it was possible to characterise the clinical features even better. Twenty‐five (74%) patients were men, with a mean age at diagnosis of 42 (17) years. The most common reason for referral was heart failure in 21 patients (62%), followed by uncertain echocardiographic findings in four (12%) and palpitations in two patients (6%). Twenty seven patients (79%) presented with shortness of breath, with 22 patients (65%) being in New York Heart Association class I and II, and 12 patients (35%) being in class III and IV. Left ventricular ejection fraction in this cohort was 33 (13)%. Most patients (94%) had an abnormal ECG, with left bundle branch block being the most common abnormality (in 44% of the patients). Six (18%) patients had familial occurrence. Embolic events were never the reason for referral in this series. However, they are known to be common complications.
When this largest series of adult patients is compared with the two largest paediatric series,12,18 the most important differences are the lack of facial dysmorphism and of Wolff‐Parkinson‐White syndrome in the adult population. This may be due to a different genetic background but with the same morphological appearance of the cardiac anomaly. Furthermore, a much lower familial occurrence in the adult population may be explained by incomplete screening of the siblings. Complete bundle branch block was more common in adults with IVNC. Other important clinical features such as symptoms of heart failure, ventricular tachycardias, systemic embolic events, pulmonary embolism or the location of non‐compacted segments were comparable between the adult and at least one of the two paediatric populations. Clinical symptoms usually depend on the extent of non‐compacted cardiac segments.
DIAGNOSIS
Non‐invasive assessment
Echocardiography
Echocardiography is considered the reference standard for the diagnosis of IVNC. To standardise and facilitate the diagnosis, we recently established four clear‐cut echocardiographic criteria (table 2).3
Table 2 Diagnostic criteria for isolated non‐compaction of the myocardium.
| 1. | Absence of coexisting cardiac abnormalities (other than 2–4) by definition |
| 2. | Typical two‐layered structure of the myocardium with a thin, compacted outer (epicardial) band and a much thicker, non‐compacted inner (endocardial) layer consisting of trabecular meshwork with deep endocardial spaces (the maximum end systolic ratio of the non‐compacted endocardial layer to the compacted myocardium of >2 is characteristic) |
| 3. | Predominant segmental location of the abnormality (that is, non‐compacted myocardium is predominantly (>80%) found in the apical and mid‐ventricular areas of both the inferior and the lateral wall). |
| 4. | Colour Doppler echocardiographic evidence of deeply perfused intertrabecular recesses (in contrast to myocardial sinusoids, intertrabecular spaces do not communicate with the coronary circulation) |
For best visual differentiation of the characteristic two‐layered structure consisting of a thin, compacted outer (epicardial) layer and a much thicker, non‐compacted inner (endocardial) layer, the parasternal short axis at end systole is used (video 1; to view video footage please visit the Heart website—http://www.heartjnl.com/supplemental).
Those criteria have now been validated against dilated cardiomyopathy, as well as valvular and hypertensive heart disease.19
The left ventricle is divided into nine segments to describe precisely the location of the affected segments: one apical, four mid‐ventricular and four basal segments (with an anterior, septal, lateral and inferior segment each).3,17 The combination of all criteria has a very high specificity for IVNC.
On the basis of the extensive experience of the principle author, the affected segments are often hypokinetic in symptomatic patients or in those patients with impaired left ventricular systolic function, in contrast with asymptomatic patients identified during family screening.
An important differential diagnostic consideration is the presence of prominent trabeculations as a common variant of normal hearts.20 These, however, most often course from the free wall to the ventricular septum.
In the case of poor image quality, contrast echocardiography clearly demarcating the endocardial borders may be helpful and may facilitate the diagnosis.6 Even better visualisation of the intertrabecular spaces can be obtained with Doppler tissue (strain rate) imaging.4
Positron emission tomography
Positron emission tomography (PET), which quantitatively evaluates myocardial blood flow and coronary flow reserve, may be used to assess microcirculatory dysfunction ultimately responsible for the wall motion abnormalities. To this end, regional myocardial blood flow at rest and during standardised pharmacological stress and coronary flow reserve were quantitatively evaluated in 12 patients with IVNC by using PET and 13N‐ammonia. A regional myocardial perfusion defect was found in nearly 60% of non‐compacted segments, whereas 90% of normal segments were not defective.21 Coronary flow reserve was decreased in 75% of non‐compacted segments but was not statistically different from coronary flow reserve in segments without non‐compaction. However, coronary flow reserve was significantly decreased in most segments with wall motion abnormalities.21 Thus, a decreased coronary flow reserve is not only confined to non‐compacted segments but extends to most of the segments with wall motion abnormalities in these patients. Although a causal relationship between microcirculatory dysfunction and wall motion abnormalities, and hence heart failure, cannot necessarily be established, microvascular dysfunction in IVNC may be responsible for the contractile impairment.
Computed tomography
Computed tomography (CT) findings of IVNC have been rarely described in the literature. Hamamichi et al22 investigated six adolescent patients by using both ultrafast CT and magnetic resonance imaging (MRI) to diagnose IVNC. CT showed early defects of varying degrees and rate enhancement of the non‐compacted ventricular myocardium. Although ultrafast CT provides high‐resolution imaging of non‐compacted myocardium, CT, unlike MRI, has not been widely used in the diagnosis of IVNC and will perhaps not be used in the future. Clear diagnostic criteria for IVNC have not been established in CT.
MRI
After some cases of adult IVNC had initially been described on the basis of cardiac MRI,7 more recently (small) patient groups have been studied. Weiss et al23 examined eight paediatric patients with echocardiographic signs of IVNC. The diagnosis could be made with MRI in six of eight patients with IVNC. Although MRI and echocardiography were well correlated in assessing compacted myocardium, results with non‐compacted myocardium were not correlated.23 However, recent technological advances have resulted in superior image quality. Recently, Petersen et al24 compared magnetic resonance images from seven patients with left ventricular non‐compaction with those of healthy controls and of patients with diseases such as hypertension or hypertrophic cardiomyopathy. They determined the ratio of non‐compacted to compacted myocardium in diastole in the 17‐segment model and found that a ratio > 2.3 accurately distinguished pathological non‐compaction. The higher value, as compared with the echocardiographic ratio to be taken in systole (table 2), reflects that it is taken in diastole. Another potential advantage of MRI is the possibility of identifying subendocardial perfusion deficits.7 Although echocardiography is the reference standard for the diagnosis of IVNC, MRI clearly has potential, especially in patients for whom good echocardiographic quality cannot be obtained.
Invasive assessment
Angiography
The typical features of IVNC have also been observed by ventriculography.8 After injection of contrast medium, the “loosened” myocardium can be identified. However, a left ventricular angiogram and coronary angiography are usually performed to rule out other concomitant cardiac abnormalities and not for the diagnosis of IVNC.
Pathoanatomical findings
IVNC is characterised by hypokinetic and hypertrophic segments of the left ventricular wall consisting of a thick non‐compacted endothelial layer and a thin compact epicardial layer (fig 1).

Figure 1 Transmural, histological section of the left ventricle (haematoxylin and eosin stain). Arrows indicate the epicardial (compacted) layer. Necroses (asterisks) are within the trabeculations of the endocardial (non‐compacted) layer but not within the epicardial zone (arrowheads). Reproduced from Oechslin EN et al17 with permission from Elsevier Science Inc.
The endocardium contains deep intertrabecular recesses and prominent trabeculations resulting from an arrest of compaction of myocardial fibres during embryogenesis (fig 2). This arrest usually occurs in week 5–8 of fetal life when the myocardium is gradually compacted and the intertrabecular spaces are transformed into capillaries. At this stage, the coronary circulation develops. Compaction during endomyocardial morphogenesis usually progresses from the epicardium towards the endocardium and from the base towards the apex.
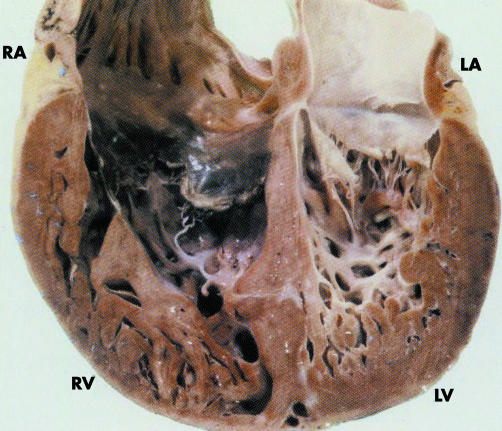
Figure 2 Transsectional view from the anterior on the dorsal half of the heart of a 21‐year‐old man with diagnosed isolated ventricular non‐compaction (IVNC). Apart from numerous trabeculations and deep recesses, note the pronounced fibroelastosis of the left ventricle (LV). LA, left atrium; RA, right atrium; RV, right ventricle. Reproduced from Oechslin EN, et al17 with permission from Elsevier Science Inc.
Biventricular non‐compaction has previously been proposed. The left ventricle is uniformly affected and the right ventricle in less than 50% of patients.13 However, in our own clinic, we no longer attempt to diagnose involvement of the right ventricle, as the exact differentiation between normal variants of the usually highly trabeculated right ventricle and pathological forms may be difficult.17
No specific histological finding has been described in IVNC, although increased subendocardial fibrosis was found in some cases. In a series of 14 cases, predominantly consisting of autopsied hearts, endocardial fibroelastosis was a characteristic histological feature.25
In IVNC, the deep intertrabecular recesses communicate with the cavity of the left ventricle but not with the coronary circulation, whereas in non‐compaction associated with other congenital heart disease (non‐isolated non‐compaction), the intertrabecular recesses communicate both with the left ventricular cavity and the coronary circulation.17
Gene defects in α dystrobrevin, Csx and FKBP12 have been associated with the non‐isolated form.
IVNC is different and distinct from left ventricular hypertrabeculation. Left ventricular hypertrabeculation has been defined as the presence of more than three prominent trabeculations in the left ventricle and is often associated with underlying extracardiac problems.26 Stöllberger et al26 therefore argue that other pathogenetic processes may be responsible for non‐compaction, which would then be an acquired disease. However, substantial evidence is now supporting the developmental hypothesis of non‐compaction.1
GENETICS
IVNC is a genetically heterogeneous disorder. A proper diagnosis of IVNC is crucial not only because of its high mortality in symptomatic patients but also for screening relatives, as familial occurrence is known. Both familial and sporadic forms have been described.
Neonatal IVNC can be a mutation caused by mutations in the G 4.5 gene located on the X chromosome, associated with Barth syndrome,27 other X‐linked infantile cardiomyopathies and X linked endocardial fibroelastosis. G 4.5 codes for taffazin, a protein that has no sequence homology with other known proteins. However, mutations in the G 4.5 gene are not the only underlying cause.
Adult forms of IVNC are genetically distinct from X‐linked infantile cases in that mutations in the G 4.5 gene were not found to be the underlying mechanism. They are suggested to be transmitted by an autosomal dominant trait.28 This is based on the observations that about one half of descendants of patients with IVNC inherit the condition and, furthermore, that cases of male to male transmission occur and that the disorder may occur in females. The adult IVNC population described by Sasse‐Klaassen et al28 had no typical signs of Barth syndrome. IVNC in adults is a distinct entity without the extracardiac manifestations seen in children. Interestingly, familial recurrence seems to be more common in adult patients with IVNC28 than in paediatric populations.12
A genome‐wide linkage analysis of a family with autosomal dominant IVNC recently showed that a gene locus maps to human chromosome 11p15.29 This was a significant further step towards the identification of a gene responsible for IVNC. However, candidate gene analysis done in the same family study did not find any alterations.
NATURAL HISTORY AND PROGNOSIS
During the six‐year follow‐up period of patients in the study by Ritter et al,13 eight of 17 patients died (three of them had a sudden cardiac death) and two underwent heart transplantation. In a larger series of patients,17 long‐term follow up (44 (39) months) showed that 35% died, half of them because of sudden cardiac death, and 12% (four patients) had undergone heart transplantation. The high incidence of both thromboembolic events (24%) and ventricular tachycardia (41%) in that series underscores the poor clinical prognosis for patients with impaired left ventricular function.
Interestingly, certain clinical characteristics have been found to be significantly more common in non‐survivors than in long‐term survivors of IVNC17: higher left ventricular end diastolic diameter at the time of initial presentation, New York Heart Association class III/IV, chronic atrial fibrillation and bundle branch block.
Because of the generally poor prognosis and because of the familial association of IVNC, first‐degree relatives should be screened by echocardiography.
TREATMENT OPTIONS
Management of patients with IVNC is similar to that of patients with other cardiomyopathies and should therefore include appropriate treatment for heart failure, management of arrhythmias and oral anticoagulation to prevent systemic emboli in patients with impaired left ventricular function.
Implantation of an internal cardioverter defibrillator system and early listing of symptomatic patients for heart transplantation have seriously to be considered. A rate of 59% of heart transplantation or death in six years of follow up has previously been reported.13 Until 2004, only seven patients with IVNC of the left ventricle were treated by heart transplantation.30
In particular, patients with a high‐risk clinical constellation for non‐survivors should be chosen for an early, more aggressive strategy.
Knowledge about aetiology, genetic background, diagnosis and prognosis of IVNC is steadily increasing as research into this, from a pathophysiological point of view, fascinating disorder has progressed enormously during the past few years. However, if standard drug treatment for heart failure is unsuccessful, cardiac transplantation is ultimately the only therapeutic possibility. This also reflects that treatment is still the weakest link in the knowledge about this disease.
To view video footage visit the Heart website—http://www.heartjnl.com/supplemental
Supplementary Material
Abbreviations
CT - computed tomography
IVNC - isolated ventricular non‐compaction
MRI - magnetic resonance imaging
PET - positron emission tomography
WHO - World Health Organization
Footnotes
Competing interests: None declared.
To view video footage visit the Heart website—http://www.heartjnl.com/supplemental
References
- 1.Freedom R M, Yoo S J, Perrin D.et al The morphological spectrum of ventricular noncompaction. Cardiol Young 200515345–364. [DOI] [PubMed] [Google Scholar]
- 2.Sedmera D, Pexieder T, Vuillemin M.et al Developmental patterning of the myocardium. Anat Rec 2000258319–337. [DOI] [PubMed] [Google Scholar]
- 3.Jenni R, Oechslin E N, Schneider J.et al Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy. Heart 200186666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams R I, Masani N D, Buchalter M B.et al Abnormal myocardial strain rate in noncompaction of the left ventricle. J Am Soc Echocardiogr 200316293–296. [DOI] [PubMed] [Google Scholar]
- 5.Weiford B C, Subbarao V D, Mulhern K M. Noncompaction of the ventricular myocardium. Circulation 20041092965–2971. [DOI] [PubMed] [Google Scholar]
- 6.Lowery M H, Martel J A, Zambrano J P.et al Noncompaction of the ventricular myocardium: the use of contrast‐enhanced echocardiography in diagnosis. J Am Soc Echocardiogr 20031694–96. [DOI] [PubMed] [Google Scholar]
- 7.Soler R, Rodriguez E, Monserrat L.et al MRI of subendothelial perfusion deficits in isolated left ventricular noncompaction. J Comput Assist Tomogr 200226373–375. [DOI] [PubMed] [Google Scholar]
- 8.Jenni R, Rojas J, Oechslin E. Isolated noncompaction of the myocardium. N Engl J Med 1999340966–967. [DOI] [PubMed] [Google Scholar]
- 9.Engberding R, Bender F. Identification of a rare congenital anomaly of the myocardium by two‐dimensional echocardiography: persistence of isolated myocardial sinusoids. Am J Cardiol 1984531733. [DOI] [PubMed] [Google Scholar]
- 10.Goebel N, Jenni R, Grüntzig A R. Persistierende myokardiale Sinusoide. Fortschr Röntgenstr 1985142692–693. [DOI] [PubMed] [Google Scholar]
- 11.Jenni R, Goebel N, Tartini R.et al Persisting myocardial sinusoids of both ventricles as an isolated anomaly: echocardiographic, angiographic, and pathologic anatomical findings. Cardiovasc Intervent Radiol 19869127–131. [DOI] [PubMed] [Google Scholar]
- 12.Chin T K, Perloff J K, Williams R G.et al Isolated noncompaction of left ventricular myocardium: a study of eight cases. Circulation 199082507–513. [DOI] [PubMed] [Google Scholar]
- 13.Ritter M, Oechslin E, Sütsch G.et al Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 19977226–31. [DOI] [PubMed] [Google Scholar]
- 14.Richardson P, McKenna W, Bristow M.et al Report of the 1995 World Health Organization/ International Society and Federation of Cardiology task Force on the Definition and Classification of Cardiomyopathies. Circulation 199693841–842. [DOI] [PubMed] [Google Scholar]
- 15.Nugent A W, Daubeney P E F, Chondros P.et al The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med 20033481639–1646. [DOI] [PubMed] [Google Scholar]
- 16.Pignatelli R H, McMahon C J, Dreyer W J.et al Clinical characterization of left ventricular noncompaction in children. Circulation 20031082672–2678. [DOI] [PubMed] [Google Scholar]
- 17.Oechslin E N, Attenhofer‐Jost C, Rojas J R.et al Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 200036493–500. [DOI] [PubMed] [Google Scholar]
- 18.Ichida F, Hamamichi Y, Miyawaki T.et al Clinical features of isolated noncompaction of the ventricular myocardium. J Am Coll Cardiol 199934233–240. [DOI] [PubMed] [Google Scholar]
- 19.Frischknecht B S, Attenhofer Jost C H, Oechslin E N.et al Validation of noncompaction criteria in dilated cardiomyopathy, and valvular and hypertensive heart disease. J Am Soc Echocardiogr 200518865–872. [DOI] [PubMed] [Google Scholar]
- 20.Boyd M T, Seward J B, Tajik A J.et al Frequency and location of prominent left ventricular trabeculations at autopsy in 474 normal human hearts: implications for evaluation of mural thrombi by two‐dimensional echocardiography. J Am Coll Cardiol 19879323–326. [DOI] [PubMed] [Google Scholar]
- 21.Jenni R, Wyss C A, Oechslin E N.et al Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J Am Coll Cardiol 200239450–454. [DOI] [PubMed] [Google Scholar]
- 22.Hamamichi Y, Ichida F, Hashimoto I.et al Isolated noncompaction of the ventricular myocardium: ultrafast computed tomography and magnetic resonance imaging. Int J Cardiovasc Imaging 200117305–314. [DOI] [PubMed] [Google Scholar]
- 23.Weiss F, Habermann C R, Lilje C.et al MRI in the diagnosis of non‐compacted ventricular myocardium (NCVM) compared to echocardiography. Fortschr Röntgenstr 20031751214–1219. [DOI] [PubMed] [Google Scholar]
- 24.Petersen S, Selvanayagam J B, Wiesmann F.et al Left ventricular noncompaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 200546101–105. [DOI] [PubMed] [Google Scholar]
- 25.Burke A, Mont E, Kutys R.et al Left ventricular noncompaction: a pathological study of 14 cases. Hum Pathol 200536403–411. [DOI] [PubMed] [Google Scholar]
- 26.Stöllberger C, Finsterer J. Left ventricular hypertrabeculation/noncompaction. J Am Soc Echocardiogr 20041791–100. [DOI] [PubMed] [Google Scholar]
- 27.Ichida F, Tsubata S, Bowles K R.et al Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation 20011031256–1263. [DOI] [PubMed] [Google Scholar]
- 28.Sasse‐Klaassen S, Gerull B, Oechslin E.et al Isolated noncompaction of the left ventricular myocardium in the adult is an autosomal dominant disorder in the majority of patients. Am J Med Genet 2003119A162–167. [DOI] [PubMed] [Google Scholar]
- 29.Sasse‐Klaassen S, Probst S, Gerull B.et al Novel gene locus for autosomal dominant left ventricular noncompaction maps to chromosome 11q15. Circulation 20041092720–2723. [DOI] [PubMed] [Google Scholar]
- 30.Stamou S C, Lefrak E A, Athari F C.et al Heart transplantation in a patient with isolated noncompaction of the left ventricular myocardium. Ann Thorac Surg 2004771806–1808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.