Although most patients with atrial fibrillation present without haemodynamic compromise, there is a small group of patients who are considerably compromised by the onset of atrial fibrillation. These patients require immediate hospitalisation and urgent intervention to prevent further deterioration.
The rate versus rhythm debate and the efficacy and safety of anticoagulation are just two examples of key management decisions the clinician and patient must face. This situation is compounded in the haemodynamically unstable patient, both by the need to act speedily and by the lack of research in this area. Although there is general agreement that such patients should be immediately hospitalised, supportive treatment (eg, oxygen) provided, and treatment of any precipitants (eg, fever and myocardial infarction) started, the decision regarding what specific atrial fibrillation treatment should be embarked on is more controversial.
Consensus statements by the Resuscitation Council (UK)1 and the American College of Cardiology/American Heart Association Task Force/European Society of Cardiology2 have given guidance on those patients considered at highest risk of haemodynamic instability—that is, those with atrial fibrillation with a ventricular rate >150 bpm, ongoing chest pain or critical perfusion. Patients with lower rates and certainly rates <120 bpm are more probably compromised by co‐morbidities, such as myocardial ischaemia, pneumonia or chronic obstructive pulmonary disease exacerbation, and treatment should be aimed at resolving these. In the setting of haemodynamic instability, concerns about intervention in the absence of anticoagulation and echocardiography are counterbalanced by the need for urgent treatment. This may include the need to treat important problems such as hypoxia, left ventricular failure, acute ischaemia, pyrexia and electrolyte disorders.
There are several specific precipitants and comorbidities that mandate specific treatments. These include primary cardiac electrophysiological abnormalities, such as Wolf–Parkinson–White syndrome, in which patients may develop ventricular rates >200 bpm with the potential for acute ventricular dysfunction, and non‐cardiac conditions, such as thyrotoxicosis, in which atrial fibrillation will not respond to any strategy that does not first treat the underlying thyroid disease.
Certain treatments are also known to be contraindicated or ineffective in the above groups. For example, the slow onset of action of digoxin makes it an inappropriate choice in haemodynamically unstable patients, and drugs which block or delay atrioventricular nodal conduction (eg, digoxin, verapamil and diltiazem) are contraindicated in patients with Wolf–Parkinson–White syndrome and other accessory pathway syndromes. If given, the atrioventricular nodal blocking action of these drugs will potentiate the ventricular response in atrial fibrillation, leading to greater haemodynamic instability. In contrast, the use of flecainide, although contraindicated in most haemodynamically unstable patients, as most will have ischaemic or structural heart disease, is appropriate in patients with Wolf–Parkinson–White syndrome.
In patients with permanent atrial fibrillation in which haemodynamic instability is associated with an unacceptably high ventricular rate the primary aim is that of rate control. In other patients with acceptable ventricular rates whose cardiac function has been compromised by onset of atrial fibrillation in the context of other cardiac abnormalities (eg, hypertensive heart disease and valvular heart disease), rate control is unlikely to bring about clinical improvement, and there is a need for the restoration of sinus rhythm.
The systematic review for this guideline found limited trial evidence to inform recommendations for the management of atrial fibrillation in patients with acute haemodynamic instability.
In patients with acute‐onset atrial fibrillation and a mean ventricular rate of 122 bpm, one retrospective observational study3 found that of the 83% who were refractory (for at least 1 h) to intravenous procainamide, 89% were successfully restored to sinus rhythm using DC cardioversion. Moreover, the rate of adverse incidents or complications was 9% v 0% for the two treatments. This study explicitly excluded patients who were judged to require immediate electrical cardioversion or intubation. Therefore, DC cardioversion was recommended in all patients with atrial fibrillation presenting with haemodynamic instability.
The use of amiodarone, which allows a fairly rapid reduction in ventricular rate in most patients, with a proportion of these reverting to sinus rhythm, is more common in UK clinical practice. The guideline development group reviewed three studies4,5,6 which determined the effectiveness of amiodarone. In these studies (n = 60), 46% reverted to sinus rhythm at 30 min,4 73% at 12 h4 and 80% at 24 h.5 A considerable proportion of patients with relatively low ventricular rates (mean heart rate 103/min) developed a paradoxical bradycardia. The one study which specifically examined the use of amiodarone in patients with proven severe cardiac dysfunction (left ventricular ejection fraction <15%) showed that 75% reverted to sinus rhythm within 30 min, with a decrease in heart rate in all patients but a non‐significant increase in cardiac index.6 Thus, it was recommended that amiodarone be used in patients when there is an unacceptable delay in providing DC cardioversion.
A third study7 assessed the efficacy of diltiazem, a rate‐limiting calcium antagonist which resulted in an effective reduction in ventricular rate in 75% of the patients studied, with 50% achieving a rate of <100 bpm and 80% achieving a rate reduction of >20%. Thus, pharmacological rate control (with β‐blockers or rate‐limiting calcium antagonists) is recommended in patients in whom haemodynamic instability is related to an excessive ventricular rate.
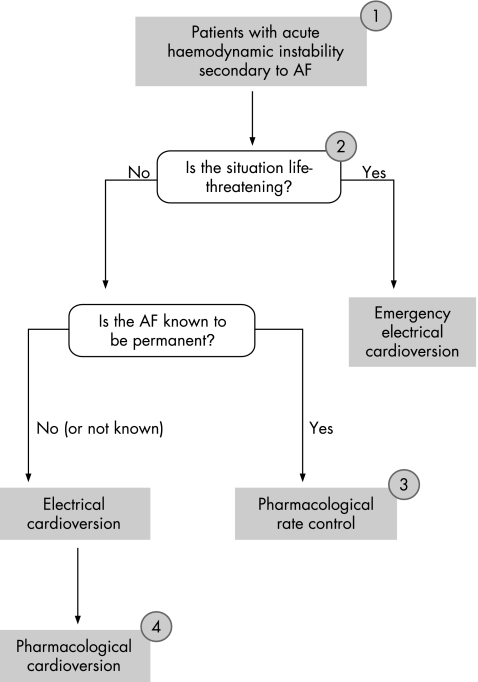
It was recognised that the evidence relating to amiodarone toxicity should inform treatment strategies and that at all times, attention should be paid to the side effect profile of any treatment used in the treatment of atrial fibrillation. The Guideline Development Group were concerned that in the absence of relevant published studies, the widespread use of amiodarone has become a practice born of custom rather than evidence. To this end, the guideline highlights that amiodarone is a second‐line choice for patients with known permanent atrial fibrillation, where rate control is the primary aim, because if it is used acutely there is an inherent risk that the drug will be continued long term and thereby expose the patient to potentially avoidable toxicity (fig 1).
Figure 1 Haemodynamically unstable atrial fibrillation (AF) treatment algorithm. (1) Diagnosis to be confirmed by electrocardiogram. Check electrolytes and review chest x ray. Attempt to establish the aetiology of acute haemodynamic instability. (2) Any emergency intervention should be performed as soon as possible and the initiation of anticoagulation should not delay any emergency intervention. (3) Where urgent pharmacological rate control is indicated, intravenous treatment should be with (a) β‐blockers or rate‐limiting calcium antagonists, (b) amiodarone, where β‐blockers or calcium antagonists are contraindicated or ineffective. (4) Where there is a delay in organising electrical cardioversion, intravenous amiodarone should be used. In those with known Wolf–Parkinson–White syndrome, flecainide is an alternative (atrioventricular node blocking agents such as diltiazem, verapamil or digoxin should not be used)
None of the identified studies dealt with the issue of anticoagulation in patients with acute haemodynamically unstable atrial fibrillation. It is known that the onset of atrial fibrillation is associated with a cluster of thromboembolic events,8 but the development of intra‐atrial thrombi and hence the immediate risk of thromboembolism is regarded as very small (but not insignificant) in the first 48 h. A study of 357 patients with symptoms indicating the onset of acute atrial fibrillation showed that thromboembolism occured in three patients in whom sinus rhythm was restored within 48 h (250 reverted spontaneously and 107 underwent cardioversion; none were anticoagulated);9 indeed, similar rates of thromboembolism have been found in other studies.10 However, intra‐atrial thrombus has been detected by transoesophageal echocardiography in 15% of patients with atrial fibrillation of <72 h duration.11
There have been no studies on patients with acute‐onset atrial fibrillation directly comparing the risk of thromboembolism with the risks of anticoagulation. Thus, it was recommended that heparin (either unfractionated or low molecular weight) be used as soon as possible. If, however, the patient was in extremis, no intervention should delay the treatment directed specifically at reverting or controlling the atrial fibrillation.
Oral anticoagulation should also be continued in patients in whom the prior duration of atrial fibrillation is unknown. When atrial fibrillation has started and sinus rhythm successfully restored within 48 h, and when the patient has no other risk factors for atrial fibrillation, recurrence and reference to the stroke risk stratification algorithm indicates anticoagulation to be unnecessary, it need not be commenced. The risk factors for recurrence of atrial fibrillation include previous recurrences of atrial fibrillation, a history of failed attempts at cardioversion, and structural heart disease (eg, mitral valve disease, left ventricular dysfunction or an enlarged left atrium).
Management of atrial fibrillation in patients postoperatively
Postoperative atrial fibrillation after cardiothoracic surgery is a major problem occurring in approximately one third of patients after coronary heart surgery.12 The occurrence of atrial fibrillation after valvular heart surgery is even higher.13 Postoperative atrial fibrillation is associated with a greater risk of mortality and morbidity.14 Furthermore, postoperative atrial fibrillation predisposes people to a considerably increased risk of stroke and thromboembolism, suggesting that patients should be anticoagulated when postoperative atrial fibrillation persists for >48 h.15,16
Although postoperative atrial fibrillation can be transient and generally self‐limiting, treatment is indicated for those patients who remain symptomatic, as well as for those who become haemodynamically unstable, or develop cardiac ischaemia or heart failure. Conventional treatment strategies have included electrical cardioversion, atrial overdrive pacing using temporary epicardial pacing leads (if atrial flutter is the dominant rhythm), pharmacological rate control and antithrombotic treatment. Cardioversion may also be attempted before discharge from hospital.
Management of medical comorbidities (eg, hypoxia) and the correction of underlying electrolyte imbalance (especially potassium and magnesium) is part of the management strategy for the prevention of postoperative atrial fibrillation.17 Most cardiothoracic units have strategies to maintain the serum potassium at >4 mmol/l and some will often endeavour to maintain the serum potassium at >4.5 mmol/l.18 One recent meta‐analysis found that giving magnesium is an effective prophylactic measure for the prevention of postoperative atrial fibrillation, but it did not markedly alter the length of stay or in‐hospital mortality.19
Currently, there is a marked variation in the management of postoperative atrial fibrillation. The National Institute for Health and Clinical Excellence (NICE) guideline investigated whether the perioperative administration of antiarrhythmic drugs is an effective prophylaxis in preventing postoperative atrial fibrillation, and in those cases where postoperative atrial fibrillation develops, which is the most effective treatment strategy.
Prophylaxis
In the prophylaxis and management of postoperative atrial fibrillation, the appropriate use of antithrombotic therapy and correction of identifiable precipitants (such as electrolyte imbalance or hypoxia) are recommended. The systematic review of this guideline identified that evidence was available for amiodarone, a β‐blocker, sotalol or rate‐limiting calcium antagonists as prophylactic treatments against postoperative atrial fibrillation, although there were a few direct comparative trials. In patients undergoing cardiothoracic surgery, the risk of postoperative atrial fibrillation should be reduced by giving one of the following: amiodarone, a β‐blocker, sotalol or a rate‐limiting calcium antagonists. In patients undergoing cardiac surgery or pre‐existing β‐blocker therapy, this treatment should be continued unless contraindications develop (such as postoperative bradycardia or hypotension).
Treatment
Unless contraindicated, a rhythm control strategy should be the initial option for the treatment of postoperative atrial fibrillation after cardiothoracic surgery. Unless contraindicated, postoperative atrial fibrillation after non‐cardiothoracic surgery should be managed in a similar manner to acute‐onset atrial fibrillation from any other precipitant.
Conclusion
The purpose of this guideline is the production of a rationale for the treatment of patients with acute atrial fibrillation. One group of patients would be those presenting to the emergency department or any other acute hospital setting in whom atrial fibrillation is associated with haemodynamic instability. As the subject of atrial fibrillation with acute haemodynamic instability has received little robust methodological study, some of the recommendations in the NICE guideline necessarily had to be extrapolated from other studies. The guideline highlights the need for thorough initial evaluation to exclude relevant comorbidites (eg, thyrotoxicosis), identify specific subgroups (eg, patients with accessory pathways) and determine how long atrial fibrillation has been present. It then directs the clinician through a clear rationale to the conclusions that properly balance the need for rapid treatment with an understanding of the risks involved. Thus, the management algorithm recommends the use of rapid‐onset but low‐toxicity modalities as preferred treatments and discourages a single “one therapy fits all” approach.
In the setting of postoperative atrial fibrillation, the NICE guideline provides recommendations for prophylaxis and treatment of atrial fibrillation (table 1).
Table 1 Recommendations for the management of acute atrial fibrillation with haemodynamic compromise.
| 1. In patients with a life‐threatening deterioration in haemodynamic stability after the onset of atrial fibrillation, emergency electrical cardioversion should be performed, irrespective of the duration of the atrial fibrillation. |
| 2. In patients with non‐life‐threatening haemodynamic instability after the onset of atrial fibrillation, the following should be considered: |
| a. electrical cardioversion |
| b. where there is a delay in organising electrical cardioversion, intravenous amiodarone should be used |
| c. in those with known Wolff–Parkinson–White syndrome: |
| –flecainide is an alternative for attempting pharmacological cardioversion |
| –atrioventricular node blocking agents (such as diltiazem, verapamil or digoxin) should not be used. |
| 3. In patients with known permanent atrial fibrillation in which haemodynamic instability is caused mainly by a poorly controlled ventricular rate, a pharmacological rate control strategy should be used. |
| 4. Where urgent pharmacological rate control is indicated, intravenous treatment should be given with one of the following: |
| a. β‐blockers or rate‐limiting calcium antagonists |
| b. amiodarone, where β‐blockers or calcium antagonists are contraindicated or ineffective. |
Footnotes
Competing interests: None.
References
- 1.Nolan J P, Deakin C D, Soar J.et al European Resuscitation Council European Resuscitation Council guidelines for resuscitation 2005. Section 4. Adult advanced life support. Resuscitation 200567(Suppl 1)S39–S86. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Lyden R E, Asinger R W.et al American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. Eur Heart J 2001221852–1923. [DOI] [PubMed] [Google Scholar]
- 3.Michael J A, Stiell I G, Agarwal S.et al Cardioversion of paroxysmal atrial fibrillation in the emergency department. Ann Emerg Med 199933379–387. [DOI] [PubMed] [Google Scholar]
- 4.Strasberg B, Arditti A, Sclarovsky S. Efficacy of intravenous amiodarone in the management of paroxysmal or new atrial fibrillation with fast ventricular response. Int J Cardiol 1985747–55. [DOI] [PubMed] [Google Scholar]
- 5.Faniel R, Schoenfeld P. Efficacy of i.v. amiodarone in converting rapid atrial fibrillation and flutter to sinus rhythm in intensive care patients. Eur Heart J 19834180–185. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A. Intravenous amiodarone therapy for atrial fibrillation and flutter in critically ill patients with severely depressed left ventricular function. South Med J 199689779–785. [DOI] [PubMed] [Google Scholar]
- 7.Wang H E, O'Connor R E, Megargel R E.et al The use of diltiazem for treating rapid atrial fibrillation in the out of hospital setting. Ann Emerg Med 20013738–45. [DOI] [PubMed] [Google Scholar]
- 8.Wolf P A, Kannel W B, McGee D L.et al Duration of atrial fibrillation and imminence of stroke: the Framingham study. Stroke 198314664–667. [DOI] [PubMed] [Google Scholar]
- 9.Weigner M J, Caulfield T A, Danias P G.et al Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hrs. Ann Intern Med 1997126615–620. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher M M, Hennessy B J, Edvardsson N.et al Embolic complications of direct current cardioversion of atrial arrhythmias: association with low intensity of anticoagulation at the time of cardioversion. J Am Coll Cardiol 200240926–933. [DOI] [PubMed] [Google Scholar]
- 11.Stoddard M F, Dawkins P R, Prince C R.et al Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transoesophageal echocardiographic study. J Am Coll Cardiol 199525452–459. [DOI] [PubMed] [Google Scholar]
- 12.Vecht R J, Nicolaides E P, Ikweuke J K.et al Incidence and prevention of supraventricular tachyarrhythmias after coronary bypass surgery. Int J Cardiol 198613125–134. [DOI] [PubMed] [Google Scholar]
- 13.Creswell L L, Schuessler R B, Rosenbloom M.et al Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 199356539–549. [DOI] [PubMed] [Google Scholar]
- 14.Almassi G H, Schowalter T, Nicolosi A C.et al Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg 1997226501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Likosky D S, Caplan L R, Weintraub R M.et al Intraoperative and postoperative variables associated with strokes following cardiac surgery. Heart Surg Forum 20047E271–E276. [DOI] [PubMed] [Google Scholar]
- 16.Maisel W H, Rawn J D, Stevenson W G. Atrial fibrillation after cardiac surgery. Ann Intern Med 20011351061–1073. [DOI] [PubMed] [Google Scholar]
- 17.Di Biasi P, Scrofani R, Paje A.et al Intravenous amiodarone vs propafenone for atrial fibrillation and flutter after cardiac operation. Eur J Cardiothorac Surg 19959587–591. [DOI] [PubMed] [Google Scholar]
- 18.Auer J, Weber T, Berent R.et al Serum potassium level and risk of postoperative atrial fibrillation in patients undergoing cardiac surgery 3. J Am Coll Cardiol 200444938–939. [DOI] [PubMed] [Google Scholar]
- 19.Miller S, Crystal E, Garfinkle M.et al Effects of magnesium on atrial fibrillation after cardiac surgery: a meta‐analysis. Heart 200591618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]