Abstract
Background and objective
Although changes in smaller vessels is the hallmark of medium‐sized and small‐vessel vasculitis, it has been suggested that large arteries of such patients may also be affected by the early atherosclerotic process because of coexisting risk factors or systemic inflammation. This study aimed to bring additional arguments supporting this hypothesis.
Design, setting and patients
50 consecutive patients with primary systemic necrotising vasculitis and 100 controls matched for age and sex underwent ultrasonic detection of plaque in three peripheral vessels (carotid and femoral arteries and abdominal aorta). Cardiovascular risk factors and inflammation (C reactive protein (CRP)) were concomitantly measured in all participants, and diagnosis of high‐risk status was defined by the presence of known history of cardiovascular disease, type 2 diabetes or 10‐year‐Framingham Risk Score ⩾20%.
Results
Patients had higher frequency of plaque than controls in the carotid arteries (p<0.05), in the aorta (p<0.01) and in the three vessels examined (p<0.001), and adjustment for high‐risk status did not confound such difference in the aorta and in the three vessels. In the overall population of patients and controls, vasculitis was associated with a higher frequency of three‐vessel plaques (p<0.05), independently of high‐risk status and CRP. In patients, the higher frequency of three‐vessel plaques was associated with high‐risk status (p<0.05) but not with CRP, or disease and treatment characteristics.
Conclusions
Small‐vessel vasculitis is associated with more frequent subclinical atherosclerosis, especially extended to multiple peripheral vessels, and such association is not entirely explained by cardiovascular risk factors and systemic inflammation.
Medium‐sized and small‐vessel vasculitis is a rare disease whose hallmark is the change in smaller vessels,1,2 but large arteries may also be altered by systemic inflammation or cardiovascular risk factors exacerbated by treatment with corticosteroids. Few previous studies have shown that flow‐mediated vasodilatation of the brachial artery was impaired in patients with small‐vessel vasculitis,3 thus proving that early dysfunction of the large artery wall is present in this disease. Moreover, such a functional change was reversed by immunosuppressive treatment, suggesting a role of inflammation in its pathogenesis.4 The objective of this study was to raise additional arguments supporting the possibility that vasculitis may participate in the early atherosclerotic process. To this end, we compared the prevalence of ultrasound‐assessed plaques in peripheral large arteries between patients with small‐vessel vasculitis and controls matched for age and sex. We also analysed the relationships of plaques with systemic inflammation, traditional cardiovascular risk factors and characteristics of vasculitis.
Methods
Participants
A cohort of 50 consecutive patients with primary systemic necrotising vasculitis, including 26 with Wegener's granulomatosis, 11 with Churg and Strauss syndrome, 8 with microscopic polyangeitis and 5 with periarteritis nodosa, was recruited between September 2002 and May 2005. Disease and treatment characteristics such as time since diagnosis, types of corticoid or other immunosuppressive treatments, current (at time of evaluation) and initial (at time of diagnosis) Birmingham Vasculitis Activity Score,5 and antineutrophil cytoplasmic antibodies were recorded for all patients. Each patient with vasculitis (termed case) was individually matched with two controls chosen from our database of 1881 people referred to the Center of Cardiovascular Preventive Medicine, Hôpital Broussais, Paris, France, for assessment of cardiovascular risk status.6 GC and MP‐B carried out the matching process, unaware of the patient's identity and atherosclerotic status, one to one, by attributing to each case two corresponding controls of the same sex, with a history of cardiovascular disease if needed, with the closest values of age (with a variation of 2 years) and with the availability of all the study parameters; if more than two participants corresponded to the matching criteria, they were randomly assigned.
Cardiovascular risk factors and systemic inflammation
Blood pressure was the average of two successive measurements on the brachial artery using the sphygmomanometer, measured with the patient in the supine position after a rest of 5 min. Hypertension was defined as blood pressure ⩾140/90 mm Hg or the use of antihypertensive drugs. Total cholesterol, high‐density lipoprotein cholesterol after precipitation of low‐density and very low‐density lipoprotein cholesterol, and triglycerides were measured enzymatically after overnight fasting, and low‐density lipoprotein cholesterol was calculated by the Friedewald formula.6 Hypercholesterolaemia was defined as total cholesterol ⩾5.18 mmol/l or as low‐density lipoprotein cholesterol ⩾3.30 mmol/l or use of lipid‐lowering drugs. Current smoking was defined as cigarette consumption within 1 month after admission. Blood glucose was measured by enzymatic methods. Diabetes mellitus was defined as fasting blood glucose ⩾7 mmol/l or use of drugs for diabetes. High‐risk status was defined according to the National Cholesterol Education Program—Adult Treatment Panel III guidelines as the presence of a known personal history of cardiovascular diseases, including all types of documented coronary, cerebrovascular or peripheral arterial disease, type 2 diabetes or a Framingham Risk Score ⩾20% at 10 years.7 Systemic inflammation was assessed by measuring C reactive protein (CRP) by a high‐sensitivity immunoassay.8
Subclinical atherosclerosis
The presence of a plaque was carefully searched for by high‐resolution ultrasound (Ultramark 5000, Philips, Les Ulis, France) in three peripheral vessels (both extracranial carotid arteries, abdominal aorta and both femoral arteries) as described previously.9 Briefly, a plaque was defined as a focal echogenic structure encroaching into the lumen >1.5 mm, and a vessel examined was considered to be a carrier of the plaque if ⩾1 such structures were present, irrespective of the location, side and number of plaques.9 The number of diseased sites was the sum of the three sites (carotid, femoral and aorta), ranging from zero to three diseased sites.9 The agreement between repeated detection of plaque ranged from 94% to 100% according to the vessel.9 In addition to plaque detection, the intima–media thickness (IMT) of the common carotid artery was measured in the far wall along at least 1 cm of length, by high‐resolution ultrasound (Ultramark 5000, Philips, Les Ulis, France), with a procedure described and validated previously.10 Briefly, the IMT image, frozen in end diastole, was analysed offline with an automated computerised program,10 and the coefficient of variation of two repeated IMT measurements was on average 4%.10
Statistical analysis
Group comparisons were carried out by a two‐sample t test for quantitative parameters and by χ2 test for qualitative variables. The null hypothesis for equality between groups was tested by the F ratio (for quantitative variables) or by the χ2 test (for qualitative variables). Log transformation was performed for statistical analyses of triglyceride and CRP because of their skewed distributions; Mann–Whitney U test was also used to verify the results. Multivariate logistic regression was used to assess independent associations between atherosclerotic plaque, disease status (case or control) and the variables associated with the three‐vessel disease in univariate analysis. The strengths of the associations were expressed by the Wald χ2 test. Statistical significance was set at p<0.05.
Results
We found no differences in cardiovascular risk factors between patients and controls, but patients had higher CRP levels (p<0.01) than controls (table 1).
Table 1 Comparison of cardiovascular risk factors and systemic inflammation between patients with vasculitis and controls.
| Characteristics | Patients (n = 50) | Controls (n = 100) | p Value |
|---|---|---|---|
| Age, years | 55 (14) | 53 (11) | 0.31 |
| Male sex, n (%) | 33 (66) | 66 (66) | 1.00 |
| Body mass index, kg/m2 | 26 (5) | 27 (5) | 0.74 |
| Hypertension, n (%) | 30 (60) | 63 (63) | 0.80 |
| Blood pressure, mm Hg | |||
| Systolic | 134 (18) | 135 (17) | 0.73 |
| Diastolic | 79 (12) | 82 (9) | 0.21 |
| Hypercholesterolaemia, n (%) | 30 (60) | 53 (53) | 0.38 |
| Blood lipids, mmol/l | |||
| Total cholesterol | 5.59 (1.21) | 5.60 (1.10) | 0.73 |
| HDL cholesterol | 1.44 (0.45) | 1.38 (0.37) | 0.43 |
| LDL cholesterol | 4.74 (1.20) | 4.83 (1.32) | 0.94 |
| Triglycerides* | 1.28 (0.79) | 1.33 (0.82) | 0.84 |
| Diabetes, n (%) | 4 (8) | 3 (3) | 0.15 |
| Blood glucose, mmol/l | 5.24 (1.28) | 5.36 (0.85) | 0.59 |
| Smoking, n (%) | 6 (12) | 21 (21) | 0.16 |
| Previous CVD, n (%) | 9 (18) | 18 (18) | 1.00 |
| Coronary heart disease, n | 6 | 10 | |
| Cerebrovascular disease, n | 2 | 5 | |
| Peripheral arterial disease, n | 1 | 3 | |
| High risk status, n (%) | 15 (30) | 28 (28) | 0.80 |
| C reactive protein*, mg/l | 5.8 (8.7) | 2.4 (2.1) |
CVD, cardiovascular disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Values are mean (SD) unless otherwise mentioned.
*Log transformed for statistical analysis.
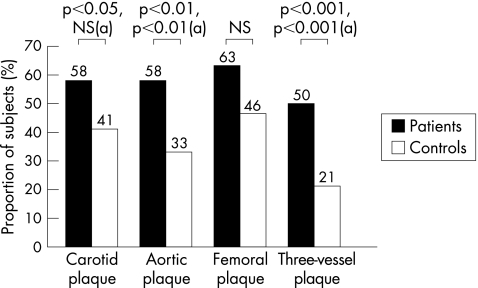
Plaques were more frequent in patients than in controls in the carotid arteries (p<0.05), aorta (p<0.01) and the three (carotid, aortic and femoral) vessels examined (p<0.001; fig 1). They also tended to be more frequent in the femoral arteries of patients than in those of controls, but the difference was not significant (p = 0.06) (fig 1). After adjustment for high‐risk status, plaques were more frequent in patients than in controls in the aorta (p<0.01) and in the three vessels (p<0.001), whereas the frequency of carotid plaques did not differ significantly between patients and controls (fig 1). Carotid IMT did not differ between patients and controls (0.60 (0.09) v 0.59 (0.09) mm).
Figure 1 Comparisons of the prevalence of atherosclerotic plaque in the carotid, aortic or femoral vessels, and those in the three vessels, between patients (n = 50) and controls (n = 100). Data are unadjusted percentages of participants with plaques in each group and p values before and after (a) adjustment for high‐risk status.
The determinants for three‐vessel plaques were analysed in cases and controls (table 2). High‐risk status was more frequent in participants with three‐vessels plaques than in those without (p<0.05), in both patients and controls (table 2). Also, CRP level was higher in those with three‐vessel plaques than in those without in the controls (p<0.05) but not in the cases (table 2). No difference in characteristics of vasculitis and type of treatment existed between patients with and without three‐vessel plaques (table 2).
Table 2 Characteristics of patients with vasculitis according to the presence or absence of three‐vessel plaque.
| Parameters | Three‐vessel plaque | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | |||||||||
| Present (n = 25) | Absent (n = 25) | p Value | Present (n = 25) | Absent (n = 25) | p Value | |||||
| High‐risk status, n (%) | 11 (44) | 4 (16) | 0.038 | 10 (48) | 18 (23) | 0.020 | ||||
| C reactive protein, mg/l* | 7.4 (11.7) | 4.3 (3.5) | 0.310 | 3.9 (3.3 | 2.0 (1.6 | 0.030 | ||||
| Disease characteristics | ||||||||||
| BVAS | ||||||||||
| At time of diagnosis | 20.3 (6.8) | 17.0 (8.1) | 0.169 | — | — | |||||
| At time of evaluation | 1.0 (1.6) | 1.0 (1.7) | 1.000 | — | — | |||||
| ANCA presence, n (%) | ||||||||||
| At time of diagnosis | 14 (56) | 12 (48) | 0.636 | — | — | |||||
| At time of evaluation | 16 (64) | 13 (52) | 0.462 | — | — | |||||
| Treatment characteristics | ||||||||||
| Current corticotherapy, n (%) | 22 (88) | 25 (100) | 0.074 | — | — | |||||
| Current corticoid dose, mg/day | 8.3 (9.1) | 8.0 (5.1) | 0.883 | — | — | |||||
| Corticotherapy duration, years | 6.5 (6.6) | 8.4 (6.1) | 0.291 | — | — | |||||
| Other immunosuppressive treatment, n(%) | ||||||||||
| Azathioprine | 9 (36) | 11 (44) | 0.558 | — | — | |||||
| Cyclophosphamide | 17 (68) | 16 (64) | 0.763 | — | — | |||||
| Hydroxychloroquine | 0 | 2 (8) | 0.091 | — | — | |||||
| Methotrexate | 4 (16) | 5 (20) | 0.711 | — | — | |||||
| Mycophenolate mofetil | 2 (8) | 2 (8) | 1.000 | — | — | |||||
ANCA, anti‐neutrophil cytoplasmic antibody; BVAS, Birmingham Vasculitis Activity Score; CHD, coronary heart disease.
Values are mean (SD) unless otherwise mentioned.
*Log transformed for statistical analysis.
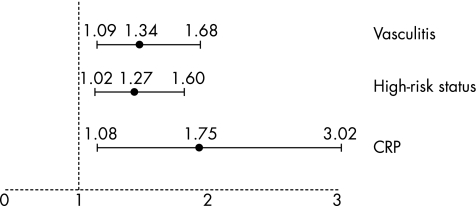
Multivariate logistic regression analysis showed that in the overall population of patients and controls, the presence of plaques in the three vessels was positively and independently associated with the presence of vasculitis (p<0.01), high‐risk status (p<0.05) and the level of CRP (p<0.05; table 3). The odds ratio of having three‐vessel plaques was 1.34 (95% confidence interval (CI) 1.09 to 1.68) by presence of vasculitis, 1.27 (95% CI 1.02 to 1.60) by presence of high‐risk status and 1.75 (95% CI 1.08 to 3.02) by each increase of 1SD in CRP level (fig 2).
Table 3 Multivariate analysis of three‐vessel plaques in the overall population of patients and controls.
| Covariates | Univariate analysis (χ2 or F ratio, p value) | Multivariate analysis (Wald χ2, p value) |
|---|---|---|
| Vasculitis (presence) | 13.0, <0.001 | 7.16, <0.01 |
| High‐risk status (presence) | 6.7, <0.01 | 4.62, <0.05 |
| C reactive protein (log transformed) | 8.3, <0.01 | 4.61, <0.05 |
Figure 2 Odds ratios with 95% confidence interval of having plaque in the three vessels examined, by presence of vasculitis or high‐risk status, or by 1SD increase in log C reactive protein (CRP).
Discussion
This case–control study shows that subclinical atherosclerosis, assessed by ultrasound in several peripheral vessels, was more frequent in patients with small‐vessel vasculitis than in controls. The magnitude of the added frequency of atherosclerotic plaque in patients with vasculitis depended on the plaque location within the arterial tree. Aortic plaque was 1.8 times as frequent and carotid plaque 1.4 times as frequent in patients with vasculitis than in controls of the same age and sex. Femoral plaque was also 1.4 times as frequent in patients than in controls, but the difference did not reach statistical significance, perhaps because of the insufficient statistical power of our small sample size owing to the rarity of the disease. Also, femoral plaque is commonplace in middle‐aged people,9 and its great prevalence in the general population may confound the effect of a particular atherogenic condition. Interestingly, the added frequency of plaque in patients with vasculitis reached the greatest magnitude—that is, 2.4 times greater frequency—when plaque affected the carotid, aortic and femoral vessels examined in the study. This finding suggests that vasculitis promotes overall the extent of subclinical atherosclerosis within the arterial tree. Contrary to plaque, carotid IMT did not differ between patients and controls, which is at odds with a previous report showing that patients with Wegener's disease had increased IMT.11 In our study, however, IMT measurement was restricted to the far wall of the common carotid segment that is usually free from intrusive atherosclerotic lesion. The so‐measured IMT may probably reflect a non‐atherosclerotic medial hypertrophic process related to hypertension rather than atherosclerosis.12 This shows that small‐vessel vasculitis is associated specifically with the atherosclerotic process and not with any subclinical arterial change of another nature.
It is important to analyse the mechanisms responsible for the association of vasculitis and atherosclerosis, especially in view of the implementation of the cardiovascular prevention strategy in patients who are carriers of this disease. A first mechanism might be the atherogenic effect of traditional cardiovascular risk factors, potentially exacerbated by the corticosteroids. This possibility is supported partly by the association between the presence of plaque in the three vessels and the presence of high‐risk status, a reflection of integrated multiple‐risk factors, in the overall population of patients and controls, as well as in the group of patients taken separately. Nevertheless, high‐risk status was not more frequent in patients with vasculitis, almost all treated with corticosteroids, than in controls, making it unlikely that corticosteroids may exacerbate atherosclerotic risk via traditional risk factors. Lastly, risk factors alone did not explain the added frequency of plaque in the aorta and in the three vessels of patients with vasculitis, which persisted after adjustment for high‐risk status. Although the analysis of independent variables associated with three‐vessel disease in the overall population may not be appropriate because patients and controls are selected groups, it enables us to prove that association between three‐vessel disease and vasculitis is independent of other factors possibly associated with atherogenesis, such as subclinical inflammation and high‐risk status of coexisting traditional cardiovascular risk factors.
An alternative possibility for explaining the higher frequency of plaque in patients with vasculitis may be the atherogenic effect of systemic inflammation,13 suggested by the independent association of CRP and plaque in the three vessels in the overall population of patients and controls. Nevertheless, such association persisted in controls taken separately but disappeared in the cases, perhaps because of the two times lesser number of patients than controls and the large standard deviation for CRP, leading to a probable type II error. Also, inflammatory markers may lose their association with atherosclerosis in patients with vasculitis having high levels of these markers, contrary to controls, in whom inflammatory markers are in the normal range. Further, vasculitis is a chronic disease subject to exacerbation and remission, so that measures of inflammatory markers vary with time and may confound their associations with atherosclerosis. Finally, vasculitis itself may exert atherogenic effects via mechanisms other than risk factors and inflammation, as suggested by the association found between vasculitis and plaque in the three vessels independently of the presence of high‐risk status and CRP level. Characteristics of the disease such as Birmingham Vasculitis Activity Score or presence of antineutrophil cytoplasmic antibodies, as well as the type of treatment, were not found to be associated, and other unmeasured factors might be involved.
Study limitations
Firstly, we included a small sample because of the rarity of the condition. There is also a danger that non‐significant associations, such as those of three‐vessel disease with CRP, are due to a type II error. Using power calculations, we can establish that the power of our statistical analysis, which failed to show a marked difference in CRP levels in patients with vasculitis with or without three‐vessel plaques, was only 31%. A second weakness of this study is the lack of measurement of other inflammatory mediators known to be involved in atherogenesis, such as soluble CD40L, interleukin (IL)18, IL8, monocyte chemoattractant protein 1, intercellular adhesion molecule 1 or soluble P‐selectin.14 Also, it would have been more appropriate to focus on a specific disease, such as Wegener's granulomatosis, rather than to use pooled patients with small‐vessel vasculitis, but the rarity of the disease does not allow recruitment of a sufficient number of patients. Lastly, our plaque estimation was purely dichotomous (presence or absence) owing to a lack of quantification of plaque size or volume, and this semiquantitative analysis might have weakened the strength of associations between atherosclerotic plaques and various markers or pathology.
In conclusion, subclinical atherosclerosis, especially extended to multiple vessels, seems to be more frequent in patients with small‐vessel vasculitis, suggesting that these patients may be at increased risk of developing atherosclerotic cardiovascular disease. Although our work does not conclude that traditional risk factors are clearly involved in the proneness of patients with vasculitis to develop atherosclerosis, the detection and treatment of cardiovascular risk factors is of crucial importance for preventing cardiovascular complications in these patients, especially in those carriers of diffuse peripheral atherosclerosis.
Abbreviations
CRP - C reactive protein
IMT - intima–media thickness
Footnotes
Competing interests: None declared.
References
- 1.Jennette J C, Falk R J. Small‐vessel vasculitis. N Engl J Med 19973371512–1523. [DOI] [PubMed] [Google Scholar]
- 2.Lhote F, Cohen P, Guillevin L. Polyarteritis nodosa, microscopic polyangiitis and Churg‐Strauss syndrome. Lupus 19987238–258. [DOI] [PubMed] [Google Scholar]
- 3.Raza K, Thambyrajah J, Townend J N.et al Suppression of inflammation in primary systemic vasculitis restores vascular endothelial function: lessons for atherosclerotic disease? Circulation 20001021470–1472. [DOI] [PubMed] [Google Scholar]
- 4.Booth A D, Jayne D R, Kharbanda R K.et al Infliximab improves endothelial dysfunction in systemic vasculitis: a model of vascular inflammation. Circulation 20041091718–1723. [DOI] [PubMed] [Google Scholar]
- 5.Luqmani R A, Bacon P A, Moots R J.et al Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med 199487671–678. [PubMed] [Google Scholar]
- 6.Simon A, Chironi G, Gariepy J.et al Differences between markers of atherogenic lipoproteins in predicting high cardiovascular risk and subclinical atherosclerosis in asymptomatic men. Atherosclerosis 2005179339–344. [DOI] [PubMed] [Google Scholar]
- 7.Anon Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Detection and evaluation. Circulation 20021063227–3234. [PubMed] [Google Scholar]
- 8.Ridker P M, Hennekens C H, Buring J E.et al C‐reactive protein and other markers of inflammation in prediction of cardiovascular disease in women. N Engl J Med 2000342836–843. [DOI] [PubMed] [Google Scholar]
- 9.Simon A, Giral P, Levenson J. Extracoronary atherosclerotic plaque at multiple sites and total coronary calcification deposit in asymptomatic men. Association with coronary risk profile. Circulation 1995921414–1421. [DOI] [PubMed] [Google Scholar]
- 10.Simon A, Gariepy J, Moyse D.et al Differential effects of nifedipine and co‐amilozide on the progression of early carotid wall changes. Circulation 20011032949–2954. [DOI] [PubMed] [Google Scholar]
- 11.de Leeuw K, Sanders J S, Stegman C.et al Accelerated atherosclerosis in patients with Wegener's granulomatosis. Ann Rheum Dis 200564753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon A, Gariepy J, Chironi G.et al Intima‐media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens 200220159–169. [DOI] [PubMed] [Google Scholar]
- 13.Ridker P M, Cushman M, Stampfer M J.et al Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997336973–979. [DOI] [PubMed] [Google Scholar]
- 14.Chironi G, Dosquet C, Del‐Pino M.et al Relationship of circulating biomarkers of inflammation and hemostasis with preclinical atherosclerotic burden in non‐smoking hypercholesterolemic men. Am J Hypertens 2006191025–1031. [DOI] [PubMed] [Google Scholar]