The ischaemic cascade1 describes the temporal sequence of pathophysiological events initiated by an imbalance between myocardial oxygen supply and demand. Depending on severity and duration, an ischaemic episode may manifest as metabolic disturbance, mechanical dysfunction, electrical changes, angina pectoris or, ultimately, as myocardial infarction. Although being the primary sequelae of coronary artery disease (CAD), quantitative relationships between myocardial perfusion changes and their consequences such as metabolic, mechanical or electrical dysfunction are not well defined. In particular, the existence of an absolute perfusion threshold preventing short‐term ischaemia has not been investigated so far.
Percutaneous coronary intervention (PCI) provides an elegant model to deal with this issue in patients with CAD. During angioplasty, myocardial blood supply is diminished below normal depending on the collateral supply from adjacent vascular regions. Absolute perfusion or myocardial blood flow (MBF, ml/min/g) can be obtained from positron emission tomography and, of late, from myocardial contrast echocardiography (MCE).2 However, only MCE is applicable during PCI owing to its mobility and short acquisition time.
Our study aimed to determine the absolute perfusion threshold that prevents myocardial ischaemia using MBF measurements by contrast echocardiography during angioplasty in humans.
Methods
Twenty eight consecutive patients with stable CAD eligible for PCI of a coronary artery without evidence of previous infarction of the target territory were included in the study. After two puffs of isosorbide dinitrate, diagnostic coronary angiography was performed via the right femoral approach. Indication for PCI was based on the visual estimate of stenosis severity and target territories were specified according to the coronary anatomy as defined by angiography. All MCE was performed with the patient in supine position using an Acuson Sequoia C256 scanner equipped with Coherent Contrast Imaging (Siemens Corp, Mountain View, California, USA) and a parallel infusion of 3 ml FS069 (OPTISON, Amersham Health SA) at a rate of 10–30 ml/h and physiological saline at a rate of 400 ml/h into the right cubital vein. Scanner settings and image quantification have been previously described.2 In each patient, target territory perfusion was measured once during angioplasty and once 15 min after angioplasty. During PCI, the intracoronary electrocardiogram was continuously recorded via the guide wire. Myocardial ischaemia was defined as the presence of ST segment elevations ⩾0.1 mV at the end of the 1‐min occlusion. The study protocol was approved by the ethics committee of the University of Bern, and all participants gave written informed consent to participate in the study.
Results
In all, 26 patients (21 men, mean 64 (standard deviation (SD) 13)) years, left ventricular ejection fraction 60% (12%)) completed the study. Two patients were excluded from the analysis due to lack of perfusion data because of a serious adverse event in one patient and poor image quality in the other. We investigated 10 antero(septal), 11 lateral and 5 inferior territories supplied by 10 left anterior descending, 11 left circumflex and 5 right coronary arteries (mean (SD) stenosis diameter reduction 76% (22%)).
During angioplasty, ST segment elevations ⩾0.1 mV were present in 19 patients (group 1), and absent in seven patients (group 2). These groups did not differ with age, sex, heart rate, blood pressure, left ventricular ejection fraction and frequency of diseased vessels (unpaired two‐group χ2 tests for nominal and Mann–Whitney U tests for continuous variables; p<0.05). Angina pectoris during angioplasty was more frequent in group 1 (58% v 0%; p = 0.008), whereas stenosis diameter reduction was more severe in group 2 (mean (SD) 88% (22%) v 71% (21%); p = 0.03).
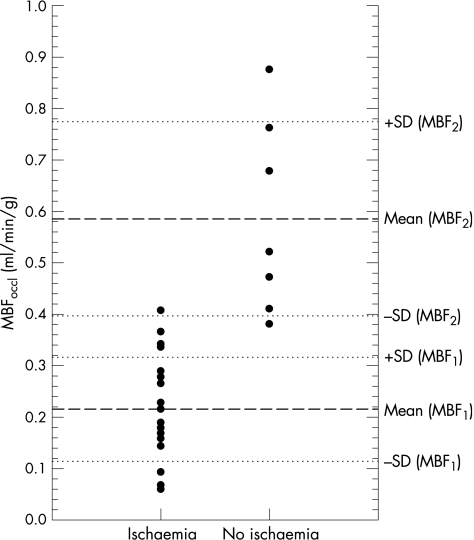
Residual MBF during PCI of group 1 (mean (SD) 0.215 (0.101) ml/min/g, range 0.060–0.408 ml/min/g) and group 2 (0.586 (0.189) ml, range 0.381–0.876 ml/min/g) differed significantly (p = 0.001) and showed only little overlap (fig 1). A threshold of 0.374 ml/min/g prevented ischaemia, with 100% sensitivity and 95% specificity (area under the curve 0.99). Baseline MBF after revascularisation varied between 0.676 and 1.773 ml/min/g (1.200 (0.340) ml/min/g), and did not differ between groups 1 and 2 (1.176 (0.354) ml/min/g v 1.265 (0.315) ml/min/g; p = 0.44).
Figure 1 Residual myocardial blood flow (MBFoccl) during angioplasty in patients with (group 1) and without (group 2) ischaemia; dashed and dotted lines indicate mean ±1 SD MBFoccl of the respective groups.
Discussion
MBF (ml/min/g) is the gold standard to quantitate myocardial blood supply. Hitherto, maximal MBF during hyperaemia has been studied extensively, whereas data on the lower limit of baseline MBF with respect to myocardial dysfunction caused by ischaemia are lacking. Previous studies on humans2,3 and the present data showed that baseline MBF varies considerably between 0.5 and 2.0 ml/min/g, thus highlighting the difficulty in defining a lower limit of normal MBF. Our data on residual MBF, however, corroborate the existence of an absolute lower perfusion limit that prevents ischaemia. This threshold of 0.374 ml/min/g is consistently below the baseline MBF of healthy volunteers and patients with CAD,2,3 and amounts to 31% of averaged baseline MBF after revascularisation. For comparison, previous studies using invasive coronary pressure and flow velocity measurements showed that a collateral relative to normal flow above 24–30% prevents myocardial ischaemia as defined by the preservation of left ventricular systolic function after myocardial infarction4 or the absence of ST segment changes during angioplasty.5
Regarding the definition of ischaemia, electrocardiogram changes as a surrogate end point are well established during angioplasty.5 Metabolic studies such as coronary blood sampling or metabolic imaging are not feasible in this setting. Regional wall motion analysis was unreliable in most cases, as we had to use atypical views showing only the target territory and adjacent segments with the patients in supine position.
Ultrasound contrast agents are pure intravascular tracers and perfusion measurements by MCE do not interfere with metabolic dysfunction that accrues early in ischaemia. Regarding the clinical implications of the presented results, we therefore suggest that MBF measurements by MCE can be used for myocardial viability studies without metabolic assessment as required by positron electron tomography.
Abbreviations
CAD - coronary artery disease
MBF - myocardial blood flow
MCE - myocardial contrast echocardiography
PCI - percutaneous coronary intervention
Footnotes
Funding: This work was supported by grants from the Swiss National Science Foundation (number 3200B0‐100065) and the Swiss Heart Foundation.
Competing interests: None.
References
- 1.Nesto R W, Kowalchuk G J. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expression of ischemia. Am J Cardiol 19785723C–30C. [DOI] [PubMed] [Google Scholar]
- 2.Vogel R, Indermühle A, Reinhardt J.et al The quantification of absolute myocardial perfusion in humans using contrast echocardiography: algorithm and validation. J Am Coll Cardiol 200545754–762. [DOI] [PubMed] [Google Scholar]
- 3.Chareonthaitawee P, Kaufmann P A, Rimoldi O.et al Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res 200150151–161. [DOI] [PubMed] [Google Scholar]
- 4.Lee C W, Park S W, Cho G Y.et al Pressure‐derived fractional collateral blood flow: a primary determinant of left ventricular recovery after reperfused acute myocardial infarction. J Am Coll Cardiol 200035949–955. [DOI] [PubMed] [Google Scholar]
- 5.Seiler C, Fleisch M, Garachemani A.et al Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol 1998321272–1279. [DOI] [PubMed] [Google Scholar]