Abstract
Objectives
To investigate diagnostic routes, echocardiographic substrates, outcomes and prognostic factors in patients with isolated ventricular non‐compaction (IVNC) identified by echocardiographic laboratories with referral from specialists and primary care physicians.
Patients and design
Since 1991, all patients with suspected IVNC were flagged and followed up on dedicated databases. Patients were divided into symptom‐based and non‐symptom‐based diagnostic subgroups.
Results
65 eligible patients were followed up for 6–193 months (mean 46 (SD 44). In 53 (82%) patients, IVNC was associated with variable degrees of left ventricular (LV) dilatation and hypokinesia, and in the remaining 12 (18%) LV volumes were normal. Diagnosis was symptom based in 48 (74%) and non‐symptom based in 17 (26%) (familial referral in 10). The non‐symptom‐based subgroup was characterised by younger age, lower prevalence of ECG abnormalities, better systolic function and lower left atrial size, whereas the extent of non‐compaction was not different. No major cardiovascular events occurred in the non‐symptom‐based group, whereas 15 of 48 (31%) symptomatically diagnosed patients experienced cardiovascular death or heart transplantation (p = 0.01, Kaplan–Meier analysis). Independent predictors of cardiovascular death or heart transplantation were New York Heart Association class III–IV, sustained ventricular arrhythmias and left atrial size.
Conclusions
IVNC is associated with a broad spectrum of clinical and pathophysiological findings, and the overall natural history and prognosis may be better than previously thought. Adult patients with incidental or familial discovery of IVNC have an encouraging outlook, whereas those who have symptoms of heart failure, a history of sustained ventricular tachycardia or an enlarged left atrium have an unstable course and more severe prognosis.
Isolated ventricular non‐compaction (IVNC) is characterised by multiple prominent trabeculations and deep intertrabecular recesses in continuity with the ventricular cavity, producing a characteristically spongy appearance of the myocardium. Since the first reported case of IVNC in 19861 and a more extensive description in 1990,2 case reports3,4,5,6,7,8 and a few studies in paediatric9,10 and adult populations11,12,13 have been published. However, IVNC remains an unclassified cardiomyopathy according to the World Health Organization classification of cardiomyopathies.14
Although presentation of IVNC has most often been reported in the context of symptoms of heart failure, other diagnostically relevant clinical manifestations (such as chest pain, ventricular arrhythmias or systemic embolic events) have been documented.12 Mounting awareness of this relatively new diagnostic entity (along with screening of relatives of affected patients9 and technical advances in echocardiography such as use of the second harmonic and contrast agents) has led to increased diagnosis of IVNC, particularly in asymptomatic patients. Current knowledge of the disease course and prognosis of IVNC is limited, especially in patients with a non‐symptomatic diagnosis. Observations based mainly on short‐term follow up of small groups of patients referred to tertiary care centres gave the impression that IVNC generally carries an ominous prognosis.12 A recent report on a larger cohort of patients suggested that the disease course may be less severe than previously thought.15 However, follow‐up studies with formal analysis of predictors of outcome are lacking.
On the basis of the supposition that IVNC is associated with a broader spectrum of clinical and morphological findings than has been described, we investigated diagnostic modalities, echocardiographic substrates and outcome in a cohort of patients with IVNC identified in the setting of echocardiographic laboratories of teaching hospitals serving patients referred both by cardiology specialists and by primary care physicians. In particular, (1) we compared outcomes of patients identified by different diagnostic routes (symptom‐based versus non‐symptom‐based diagnosis); and (2) we searched for possible prognostic factors.
METHODS
Setting and identification of patients
The study cohort was identified by the dedicated databases (set up in Bologna in 1991 and in Rotterdam in 1995) of two echocardiographic laboratories based in teaching hospitals that also provide reference centres for primary care physicians in broad regional territories. Since 1991, records of all examined non‐paediatric patients found at any time to have echocardiographic features suggestive of IVNC (that is, pronounced trabeculations and intertrabecular recesses) were individually flagged in each database. Families were not systematically screened, but patients were informed of the hereditary basis of the disease and offered the possibility of clinical evaluation for relatives. During 2004 echocardiographic diagnosis of IVNC was systematically reviewed in each centre by three independent experts in echocardiography (MF, GR and CR; EB, FJtC and KC): patients with potential ambiguities (as judged by any of the experts) were recalled for updated imaging examinations with second‐harmonic or contrast echocardiography, or both. Final diagnosis of IVNC was made according to the following criteria:
An excessively thickened myocardial wall with a two‐layered structure comprising a thin compacted layer on the epicardial side and a much thicker non‐compacted layer of prominent trabeculations and deep intertrabecular recesses on the endocardial side (fig 1). The thickness ratio of non‐compacted to compacted myocardium was measured at the site of maximum thickness during the end systolic phase of cardiac cycle for optimal visualisation of the two layers. A ratio > 2 was considered diagnostic for IVNC.8,12,15,16,17
Colour Doppler evidence that the deep intertrabecular recesses are in communication with the ventricular cavity.
Absence of coexisting cardiac anomalies.
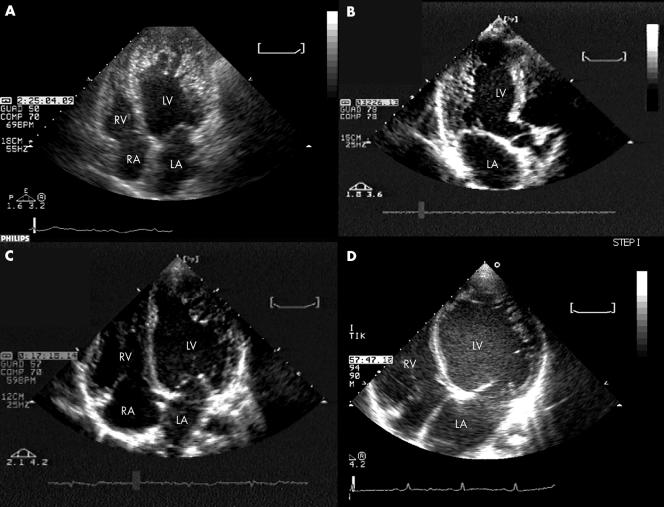
Figure 1 Representative examples at two‐dimensional echocardiography of the spectrum of left ventricular (LV) functional and morphological impairment in isolated ventricular non‐compaction. (A) Non‐compaction involving the entire apex in a patient with normal end diastolic LV volume (with an overall picture that can mimic apical hypertrophic or obliterative cardiomyopathy); (B) non‐compaction limited to the posterior wall in a patient with nearly normal end diastolic LV; (C) non‐compaction involving the apicolateral wall in a patient with mildly dilated cardiomyopathy; (D) non‐compaction involving the apicolateral wall in a patient with severely dilated cardiomyopathy. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The study was planned and performed according to the principles of the Declaration of Helsinki. All patients provided written informed consent for anonymous publication of the study data.
Instrumental examinations
Two‐dimensional and Doppler echocardiography was routinely performed according to the recommendations of the American Society of Echocardiography,18 with measurements being expressed as absolute values. Selected patients (see above) underwent contrast echocardiography for enhanced endocardial border detection with Sonovue (Bracco, Milan, Italy) as the contrast agent. All examinations included standard parasternal (long‐ and short‐axis) and apical (four‐, two‐ and three‐chamber) views, as well as M mode and two‐dimensional views. Both systolic and diastolic LV function were evaluated: LV volumes and LV ejection fraction (LVEF) were calculated at apical four‐ and two‐chamber views by the Simpson biplane method; diastolic function was assessed by the LV inflow pattern at the tip of the mitral leaflets and by the pulmonary venous flow at pulsed‐wave Doppler. A 16‐segment LV model was used to define the location of areas of non‐compacted myocardium.
On the basis of our institutional protocols for myocardial diseases, coronary angiography was routinely performed in all patients over 30 years to exclude associated coronary artery disease. In addition to routine 12‐lead resting ECG, all patients received Holter monitoring within six months of the initial evaluation. Non‐sustained ventricular tachycardia was defined as a run of more than three ventricular extrasystoles lasting less than 30 s; sustained ventricular tachycardia was defined as a ventricular run longer than 30 s.
Treatment
During the study period, warfarin (international normalised ratio 2–3) was given to patients with LV dysfunction (LVEF < 30%) or atrial fibrillation, or a history of thromboembolic episodes. Treatment for heart failure and LV dysfunction was based on current clinical guidelines19 An implantable cardioverter‐defibrillator (ICD) was implanted in patients with a history of sustained ventricular tachycardia (n = 4) or in patients with heart failure and LVEF < 30% for primary prevention of sudden death (n = 4, two of whom also had a biventricular pacing function). Biventricular pacing was implanted in the other three patients with heart failure despite optimal medical treatment.
Follow‐up data collection
Follow‐up data were obtained by planned six‐monthly visits. If patients did not attend planned visits, their families were reminded by telephone. In the event of hospitalisation (or death), causes were determined by examining the appropriate clinical records (or death certificates). Standard definitions for clinical events were used; sudden death was defined as an unexpected death occurring within 1 h of the onset of acute symptoms.
Statistical analysis
Unless otherwise stated, variables are expressed as mean (SD) or number (%). Patients were divided into two predetermined subgroups based on the diagnostic route: symptom‐based or non‐symptom‐based diagnosis. Student's t test for unpaired data was used for group comparisons. χ2 analysis was used for nominal data. Events were hospitalisation for heart failure, systemic thromboembolism, sustained arrhythmias, syncope, appropriate ICD interventions, heart transplantation and cardiovascular death. Duration of follow up was calculated from the time of first evaluation in our institutions until April 2005. Kaplan–Meier analysis was based on the combined end point of cardiovascular death or heart transplantation. Predictors of cardiovascular death and heart transplantation were assessed by a Cox multivariate model including variables that reached significance (p < 0.05) at univariate analysis. SPSS software (SPSS Inc, Chicago, Illinois, USA) was used for analysis.
RESULTS
The diagnostic criteria for IVNC were fulfilled by 65 patients (49 in Bologna and 16 in Rotterdam). All patients had comprehensive baseline and follow‐up data (no patient was lost to follow up). Table 1 reports their main clinical, ECG and echocardiographic characteristics. Contrast echocardiography was used in 27 patients. The prevalent location of non‐compaction was apicolateral (in 60 (92%) patients). In 53 (82%) patients, IVNC was associated with variable degrees of LV dilatation and hypokinesia, and in the remaining 12 (18%) patients LV volumes were normal (fig 1).
Table 1 Baseline clinical, ECG and echocardiographic characteristics according to modality of diagnosis.
| Overall (n = 65) | Symptom‐based diagnosis (n = 48) | Non‐symptom‐based diagnosis (n = 17) | p Value* | |
|---|---|---|---|---|
| Age at first evaluation (years) | 45 (16) | 49 (15) | 35 (15) | 0.002 |
| Age at initial diagnosis of myocardial disease (years) | 42 (17) | 45 (17) | 34 (16) | 0.023 |
| Familial occurrence | 20 (31%) | 8 (17%) | 12 (71%) | NA |
| Neuromuscular disease | 6 (9%) | 3 (6%) | 3 (18%) | 0.36 |
| Left bundle branch block | 21 (32%) | 20 (42%) | 1 (6%) | 0.016 |
| Normal ECG | 8 (12%) | 3 (6%) | 5 (29%) | 0.04 |
| LV end diastolic diameter (mm) | 67 (11) | 69 (12) | 62 (11) | 0.04 |
| LV ejection fraction (%) | 31 (11) | 28 (10) | 39 (11) | <0.001 |
| Left atrial size (mm) | 47 (10) | 50 (9) | 41 (8) | <0.001 |
| Moderate or severe mitral regurgitation | 35 (54%) | 29 (60%) | 6 (35%) | 0.13 |
| Number of non‐compacted segments | 6 (3) | 5 (3) | 6 (2) | 0.2 |
| Ratio of non‐compacted to compacted segments | 2.7 (0.7) | 2.8 (0.7) | 2.7 (0.6) | 0.6 |
| Transmitral restrictive pattern | 21 (32%) | 19 (40%) | 2 (12%) | 0.07 |
Data are mean (SD) or number (%).
*Comparison between subgroups with symptom‐based and non‐symptom‐based diagnosis.
LV, left ventricular.
Of note, the association between normal LV volumes and apically located non‐compaction initially suggested hypertrophic or obliterative cardiomyopathy (fig 1A).
The diagnostic route was symptom based in 48 (74%) patients: heart failure in 40 of 48 (83%) (including New York Heart Association (NYHA) class III–IV in 21); palpitation in three (6%); syncope in two (4%); chest pain in two (4%); and sustained ventricular arrhythmia in one (2%). In the remaining 17 of 65 (26%) patients, diagnosis was non‐symptom based: familial referral in 10 of 17 (59%); ECG abnormalities in four (23%), including one instance of ventricular pre‐excitation; raised creatine kinase concentration in two (12%); and cardiac murmur in one (6%). All but one of the 65 patients were in sinus rhythm at diagnosis; one patient with symptom‐based diagnosis had permanent atrial fibrillation. All 21 patients in NYHA class III–IV had systolic dysfunction (LVEF < 45%).
One patient with symptom‐based diagnosis presented a dysmorphic facial appearance in the absence of any apparent family history of IVNC. At diagnosis, six patients had associated neuromuscular disorders (muscular dystrophies, including one case of Becker's dystrophy and one limb‐girdle muscular dystrophy). No patient had an implanted device at diagnosis.
Of note, the clinical evaluations offered to patients' relatives (27 people were examined) also led to diagnosis of five cases of dilated cardiomyopathy without IVNC (in addition to the 10 identified cases of IVNC mentioned above).
Patients with non‐symptom‐based diagnosis were younger, a higher prevalence of a family history of cardiomyopathy, a lower prevalence of ECG abnormalities, better LV systolic function and smaller left atrial size. Notably, there were no apparent difference in numbers of non‐compacted segments or in the ratio of non‐compacted to compacted segments.
Duration of follow up ranged from 6–193 months (median 21 months; mean 46 (SD 44)). The duration of follow up was not significantly different between the subgroups of patients with symptom‐based and non‐symptom‐based diagnoses (40 (44) v 50 (55) months, p = 0.45). Regarding treatment during the entire observation period, in the symptom‐based diagnostic subgroup, β blockers were given to 29 (60%) patients, angiotensin‐converting enzyme inhibitors to 41 (85%), diuretics to 38 (79%), spironolactone to 19 (39%) and warfarin to 30 (62%). In the non‐symptom‐based subgroup, β blockers were given to two (12%) patients, angiotensin‐converting enzyme inhibitors to five (29%) and warfarin to four (23%). Eleven patients, all in the symptom‐based diagnostic subgroup, received implantable devices (ICD only, n = 6; biventricular pacemaker only, n = 3; biventricular pacemaker with ICD, n = 2). As table 2 shows, only one cardiovascular event (hospitalisation due to heart failure) was recorded in the subgroup of patients with non‐symptom‐based diagnosis (one patient died from an externally caused accident). In contrast, hospitalisation due to heart failure and other major cardiovascular events was common among patients with symptom‐based diagnosis, six (13%) of whom died from heart failure or sudden death. Of note, two of the eight patients fitted with an ICD received appropriate defibrillation (at 24 and 55 months after implantation).
Table 2 Events during follow up.
| Overall (n = 65) | Symptom‐based diagnosis (n = 48) | Non‐symptom‐based diagnosis (n = 17) | |
|---|---|---|---|
| Hospitalised for heart failure | 22 (34%) | 21 (44%) | 1 (6%) |
| Sustained VT | 4 (6%) | 4 (8%) | 0 |
| Syncope | 3 (5%) | 3 (6%) | 0 |
| Permanent atrial fibrillation | 6 (9%) | 6 (13%) | 0 |
| Systemic thromboembolism | 3 (5%) | 3 (6%) | 0 |
| Implantation | |||
| ICD only | 6 (9%) | 6 (13%) | 0 |
| Biventricular pacemaker only | 3 (5%) | 3 (6%) | 0 |
| Biventricular pacemaker with ICD | 2 (3%) | 2 (4%) | 0 |
| Heart transplantation | 9 (14%) | 9 (19%) | 0 |
| Cause of death | |||
| Heart failure | 3 (5%) | 3 (6%) | 0 |
| Sudden death | 3 (5%) | 3 (6%) | 0 |
| Non‐cardiovascular | 1 (2%) | 0 (0%) | 1 (6%) |
| Total deaths | 7 (11%) | 6 (13%) | 1 (6%) |
| Cardiovascular death plus heart transplantation | 15 (23%) | 15 (31%) | 0 |
HT, heart transplantation; ICD, implantable cardioverter‐defibrillator.
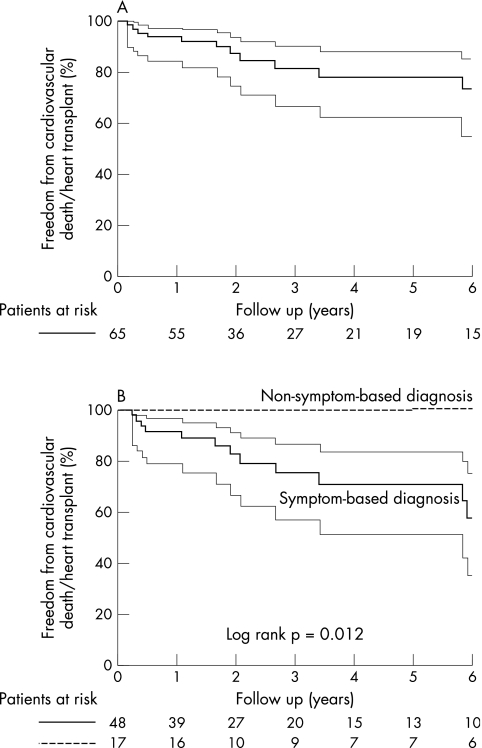
Figure 2 shows freedom from cardiovascular death or heart transplantation in the overall cohort and according to the two diagnostic subgroups (the annual event rate in the overall population was 6%). Patients with a non‐symptom‐based diagnosis had a significantly better outcome (p = 0.01). Table 3 reports analysis of candidate predictors of death or heart transplantation among all patients: NYHA class III–IV, history of sustained ventricular arrhythmias and left atrial size were independent factors. Diagnostic route was significant only at univariate analysis. Notably, neither age (at index evaluation and initial diagnosis of myocardial disease) nor greater than average number of non‐compacted segments approached significance.
Figure 2 Freedom from cardiovascular death or heart transplantation (A) in the overall cohort and (B) according to the two diagnostic subgroups. The thinner continuous lines represent 95% confidence limits.
Table 3 Analysis of candidate predictors of death or heart transplantation in the study population.
| Variables at presentation | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| NYHA class III–IV | 5.8 (2.0 to 17.0) | 0.001 | 10.9 (2.8 to 41.6) | 0.0001 |
| Sustained ventricular arrhythmias | 6.1 (1.6 to 23.1) | 0.008 | 10.1 (2.1 to 47.4) | 0.004 |
| Left atrial size (cm) | 2.5 (1.5 to 4.4) | 0.001 | 3.7 (1.8 to 7.6) | 0.0001 |
| Non‐symptom‐based diagnosis | 0.1 (0.01 to 2.4) | 0.04 | ||
| Restrictive pattern | 2.7 (0.9 to 7.9) | 0.06 | ||
| LV ejection fraction | 0.9 (0.8 to 1.0) | 0.08 | ||
| Permanent atrial fibrillation | 3.3 (0.7 to 15.2) | 0.12 | ||
| Left bundle branch block | 0.50 (0.1 to 1.6) | 0.24 | ||
| Syncope | 1.9 (0.5 to 7.2) | 0.30 | ||
| Age at first evaluation | 1.06 (0.98 to 1.05) | 0.34 | ||
| Age at initial diagnosis of myocardial disease | 1.007 (0.98 to 1.04) | 0.60 | ||
| Male sex | 1.5 (0.5 to 4.9) | 0.44 | ||
| Number of non‐compacted segments >5 | 0.4 (0.2 to 1.9) | 0.60 | ||
HR, hazard ratio; LV, left ventricular; NYHA, New York Heart Association.
DISCUSSION
We report (to our knowledge) the largest available follow‐up study of adult IVNC patients, including the first formal analysis of prognostic factors. Unlike previously reported cohorts1,2,3,4,5,6,7,8,9,10,11,12,13,14,15 recruited by inpatient or outpatient clinics, which generally focused on patients with a symptomatic diagnosis, the participants enrolled in the present study were identified by an echocardiography laboratory also serving non‐symptomatic patients. Our data indicate that the spectrum of presentation of IVNC can be wider than was previously thought. Moreover, prognosis is not necessarily ominous, especially for patients with a non‐symptom‐based initial diagnosis.
Early reports of a disease generally are of dramatic cases, whereas subsequent studies progressively broaden our picture of the affected population, extending it to patients with less and less severe disease. IVNC was first described in highly symptomatic patients affected by heart failure, sustained ventricular tachycardias or thromboembolic complications.1,4,7,9,11,12 A recent report of adult patients from a single referral centre has provided a less severe image of the disease.15 The present study confirms and extends this revised picture and provides a first formal analysis of outcome.
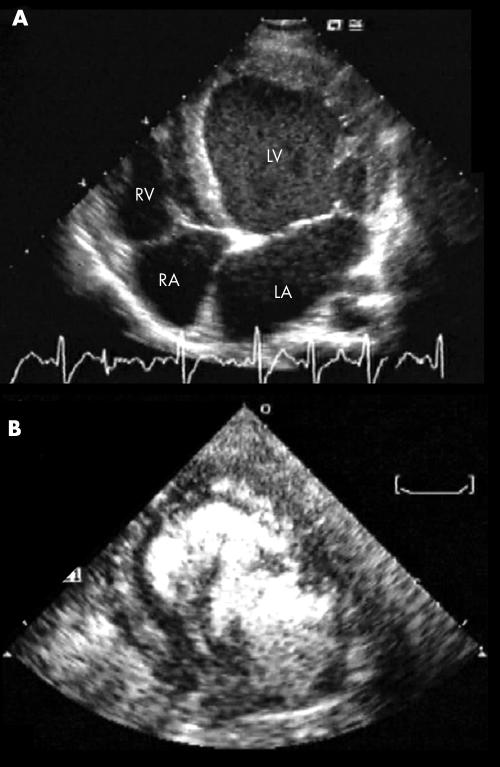
Recognition of IVNC is not always straightforward. In about a quarter of our patients, the final diagnosis of IVNC required second‐harmonic techniques and contrast echocardiography (fig 3). In a dedicated study (by one of our centres), systematic use of contrast echocardiography enhanced diagnostic sensitivity and specificity.20 In the present study, both the number of non‐compacted segments (median 6) and their more common apicolateral location reflected findings from previous studies.11,12,15,21 Although application of restrictive selection criteria reduces the risk of false‐positive cases, it may exclude anatomically mild cases of this disease, which is characterised by a wide spectrum of morphogenetic myocardial abnormalities.22 Dilated cardiomyopathy is not the only possible substrate of IVNC, as a spectrum of functional conditions can be observed,8,10,15 including pseudohypertrophic or obliterative cardiomyopathy (fig 1).
Figure 3 Example of the often decisive role of contrast echocardiography in the diagnosis of isolated ventricular non‐compaction: at two‐dimensional echocardiography in the four‐chamber view, a morphologically ambiguous picture (A) is clarified by use of the echo‐contrast medium (B), which accentuates the true trabecular pattern. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
All the major diagnostic pathways commonly encountered in clinical practice were represented in our series of patients. Heart failure was the most common symptomatic presentation, followed by sustained or paroxysmal arrhythmias and thromboembolic episodes. About one fifth of the patients had an incidental diagnosis or were identified through family referral. At diagnosis, the patients in this heterogeneously identified subgroup were younger, had a lower prevalence of ECG alterations and had less severe LV functional impairment in terms of ventricular or atrial dilatation and LVEF. These differences can be largely attributed to an earlier phase of a shared disease. However, it cannot be excluded that some of the patients with a non‐symptom‐based diagnosis might have different (intrinsically less severe) forms of this relatively undefined nosographic entity. Genotype–phenotype correlation studies could address this question. Of note, the superior LV function in patients with non‐symptom‐based diagnosis was not accompanied by less extensive non‐compaction (as assessed both by absolute numbers of segments and by the ratio of non‐compacted to compacted segments); this observation is in line with the concept that non‐compaction is probably not the cause of LV dysfunction but rather a marker of an underlying diffuse cardiomyopathy.23
As in studies where family screening was systematic,15 IVNC and classic idiopathic dilated cardiomyopathy coexisted within pedigrees. Both our diagnostic subgroups included patients with associated neuromuscular disease (Duchenne's, Becker's or myotonic dystrophy), but in the absence of systematic neurological evaluation the overall recorded prevalence of 9% may not reflect the real frequency of this problem. Taken together, these observations support the concept that IVNC is not a specific cardiomyopathy.9,15 It may be considered a subtype of genetically determined cardiomyopathy that can herald more widespread muscular disease, including dystrophinopathies and mitochondrial (cardio)myopathies.13,24,25
Within the overall cohort of patients, prognosis was rather encouraging, with freedom from cardiovascular death or heart transplantation at three and five years of about 85% and 75%, respectively. In particular, rates of sudden death and thromboembolic events were both remarkably low. As regards sudden death, it should be noted that over 15% of our patients carried an ICD or a biventricular pacemaker, or both. The low occurrence of thromboembolic events (5%) in our series (as compared with 21–38% reported in studies with similar average follow‐up durations2,12) can be attributed to our systematic administration of warfarin to patients with LV dysfunction (LVEF < 35%), atrial fibrillation or thromboembolic episodes. Of note, we did not observe thromboembolic events in patients with LVEF > 30%, even if warfarin was not given.
Our analysis indicates that the diagnostic pathway can be an important element of clinical evaluation. Incidental and familial discovery of IVNC was associated with a high probability of a stable course at least in the first few years after diagnosis. Although this finding can readily be attributed to the lead‐time effect (bias), it is clinically relevant, as it attenuates the perceived aggressiveness of the disease and provides clinicians with new clues to modulate therapeutic interventions.
This study also provides a first analytical exploration of potential prognostic factors. Our results suggest that both clinical and pathophysiological variables may help predict outcome. At multivariate analysis, clinical predictors of death or need for heart transplantation were NYHA class at first observation and history of sustained ventricular tachycardia, whereas left atrial diameter was the only echocardiographic predictor. The inability of LVEF or diameters to stratify risk can be attributed to the presence of LV systolic dysfunction in the vast majority (over 90%) of our patients. In IVNC, as in other forms of dilated cardiomyopathy,26 left atrial size may provide relevant prognostic information; the left atrium not only reflects the current burden on the LV but also offers a record of its diastolic and systolic functional history and of the presence and severity of coexistent mitral regurgitation.
Study limitations
This work provides a retrospective analysis of a prospectively constructed database. Although this analysis regards the largest reported series of patients with IVNC, the small absolute numbers limited the survival curve analysis. Although the comparison of diagnostic routes provides a valuable clinical message, it is not informative about the pathophysiological time sequence of the disease. The heterogeneous diagnostic routes may simply be associated with different phases of a single disease, but they may also reflect genetic heterogeneity. The analysis of prognostic factors should be interpreted with caution due to inevitable limitations in numbers (of patients and events) and follow‐up time, as well as the retrospective analysis. As regards patient selection, enrolment through echocardiography laboratories rather than inpatient or outpatient clinics distinguishes the present study from previous works.14,17 Nevertheless, our findings cannot be taken to reflect the prevalence, characteristics and outcome of IVNC in the general population (where the disease may eventually turn out to be even less severe). As our study design did not contemplate any systematic family screening, the results do not provide information on the genetic aspects of the disease.
Conclusions
This study indicates that IVNC can be associated with a broad spectrum of clinical and pathophysiological findings and strengthens the concept that the overall natural history and prognosis of IVNC may be better than previously thought.15 In particular, the diagnostic pathway may be an important element of clinical evaluation, with incidental and familial discovery of IVNC being associated with a high probability of a stable course for several years. Conversely, patients who have symptoms of heart failure, a history of sustained ventricular tachycardia or an enlarged left atrium have an unstable clinical course and a more severe prognosis.
ACKNOWLEDGEMENTS
We are grateful to Robin M T Cooke for writing assistance.
Abbreviations
HT - heart transplantation
ICD - implantable cardioverter‐defibrillator
IVNC - isolated ventricular non‐compaction
LV - left ventricular
LVEF - left ventricular ejection fraction
NYHA - New York Heart Association
Footnotes
Competing interests: None declared.
References
- 1.Jenni R, Goebel N, Tartini R.et al Persisting myocardial sinusoids of both ventricles as an isolated anomaly: echocardiographic, angiographic, and pathologic anatomical findings. Cardiovasc Intervent Radiol 19869127–131. [DOI] [PubMed] [Google Scholar]
- 2.Chin T K, Perloff J K, Williams R G.et al Isolated noncompaction of left ventricular myocardium: a study of eight cases. Circulation 199082507–513. [DOI] [PubMed] [Google Scholar]
- 3.Agmon Y, Connolly H M, Olson L J.et al Noncompaction of the ventricular myocardium. J Am Soc Echocardiogr 199912859–863. [DOI] [PubMed] [Google Scholar]
- 4.Jenni R, Rojas J, Oechslin E. Isolated noncompaction of the myocardium. N Engl J Med 1999340966–967. [DOI] [PubMed] [Google Scholar]
- 5.Maltagliati A, Pepi M. Isolated noncompaction of the myocardium: multiplane transesophageal echocardiography diagnosis in an adult. J Am Soc Echocardiogr 2000131047–1049. [DOI] [PubMed] [Google Scholar]
- 6.Conraads V, Paelinck B, Vorlat A.et al Isolated non‐compaction of the left ventricle: a rare indication for transplantation. J Heart Lung Transplant 200120904–907. [DOI] [PubMed] [Google Scholar]
- 7.Yasukawa K, Terai M, Honda A.et al Isolated noncompaction of ventricular myocardium associated with fatal ventricular fibrillation. Pediatr Cardiol 200122512–514. [DOI] [PubMed] [Google Scholar]
- 8.Bax J J, Lamb H J, Poldermans D.et al Non‐compaction cardiomyopathy‐echocardiographic diagnosis. Eur J Echocardiogr 20023301–302. [PubMed] [Google Scholar]
- 9.Ichida F, Hamamichi Y, Miyawaki T.et al Clinical features of isolated noncompaction of the ventricular myocardium: long‐term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 199934233–240. [DOI] [PubMed] [Google Scholar]
- 10.Pignatelli R H, McMahon C J, Dreyer W J.et al Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation 20031082672–2678. [DOI] [PubMed] [Google Scholar]
- 11.Ritter M, Oechslin E, Sutsch G.et al Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 19977226–31. [DOI] [PubMed] [Google Scholar]
- 12.Oechslin E N, Attenhofer Jost C H, Rojas J R.et al Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 200036493–500. [DOI] [PubMed] [Google Scholar]
- 13.Stollberger C, Winkler‐Dworak M, Blazek G.et al Left ventricular hypertrabeculation/noncompaction with and without neuromuscular disorders. Int J Cardiol 20049789–92. [DOI] [PubMed] [Google Scholar]
- 14.Richardson P, McKenna W, Bristow M.et al Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 199693841–842. [DOI] [PubMed] [Google Scholar]
- 15.Murphy R T, Thaman R, Blanes J G.et al Natural history and familial characteristics of isolated left ventricular non‐compaction. Eur Heart J 200526187–192. [DOI] [PubMed] [Google Scholar]
- 16.Jenni R, Oechslin E, Schneider J.et al Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy. Heart 200186666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengupta P P, Mohan J C, Mehta V.et al Comparison of echocardiographic features of noncompaction of the left ventricle in adults versus idiopathic dilated cardiomyopathy in adults. Am J Cardiol 200494389–391. [DOI] [PubMed] [Google Scholar]
- 18.Schiller N B, Shah P M, Crawford M.et al Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 19.Hunt S A, Baker D W, Chin M H.et al ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation 20011042996–3007. [DOI] [PubMed] [Google Scholar]
- 20.De Groot‐de Laat L E, Krenning B J, ten Cate F J.et al Usefulness of contrast echocardiography for diagnosis of left ventricular noncompaction. Am J Cardiol 2005951131–1134. [DOI] [PubMed] [Google Scholar]
- 21.Boyd M T, Seward J B, Tajik A J.et al Frequency and location of prominent left ventricular trabeculations at autopsy in 474 normal human hearts: implications for evaluation of mural thrombi by two‐dimensional echocardiography. J Am Coll Cardiol 19879323–326. [DOI] [PubMed] [Google Scholar]
- 22.Burke A, Mont E, Kutys R.et al Left ventricular noncompaction: a pathological study of 14 cases. Hum Pathol 200536403–411. [DOI] [PubMed] [Google Scholar]
- 23.Jenni R, Wyss C A, Oechslin E N.et al Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J Am Coll Cardiol 200239450–454. [DOI] [PubMed] [Google Scholar]
- 24.Wald R, Veldtman G, Golding F.et al Determinants of outcome in isolated ventricular noncompaction in childhood. Am J Cardiol 2004941581–1584. [DOI] [PubMed] [Google Scholar]
- 25.Chung T, Yiannikas J, Lee L C.et al Isolated noncompaction involving the left ventricular apex in adults. Am J Cardiol 2004941214–1216. [DOI] [PubMed] [Google Scholar]
- 26.Rossi A, Cicoira M, Zanolla L.et al Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol 2002401425–1430. [DOI] [PubMed] [Google Scholar]