Hypertrophic cardiomyopathy (HCM) carries a relatively high risk of sudden death, particularly in young people.1,2 To date, the clinical significance of heart rate variability (HRV) in patients with HCM is still controversial.3,4 Our study aimed to analyse HRV in a relatively large cohort of children and young patients with HCM, and to assess potential correlations with clinical markers of sudden death.
Methods
In all, 53 patients diagnosed with HCM between the ages of 18 and 40 years and body surface area‐matched controls were included in this study. The diagnosis of HCM was based on echocardiographic evidence of increased wall thickness, 2 standard deviations (SD) or more compared with age, sex and body surface area‐matched people. Maximal ventricular wall thickness (MWT) was defined as the greatest thickness in any segment and measured as the absolute value and as Z score. At the time of the study, age of patients ranged from 1 to 220 months (mean 75.6 (SD 73.7)). Patients with HCM underwent physical examination, rest electrocardiography, conventional echocardiography, exercise test and 24‐h Holter electrocardiography. HRV time‐domain analysis included the following indices: SD from the mean of the normal relative risk (RR) intervals; SD of 5‐min mean RR intervals; the mean of 5‐min SDs of RR intervals; square root of the mean squared differences of successive RR intervals; the portion of adjacent RR intervals >50 ms different; spectral power 24 h; minimum spectral power and maximum spectral power. HRV frequency‐domain analysis allowed the identification of a low‐frequency component (LF, ms) and high‐frequency peak centred around the respiratory frequency (HF, ms), and their ratio (LF/HF).
Data were expressed as mean (SD). Differences between continuous and categorical variables were assessed by unpaired Student's t test and the χ2 test, respectively. Significant results were verified using the non‐parametric Mann–Whitney U test when appropriate. A logistic regression analysis evaluated independent predictors of fatal events. Differences were considered to be significant at p<0.05.
Results
Both spectral and time‐domain analysis were significantly impaired in patients with HCM compared with controls. HRV indices were reduced in patients with syncope (n = 9; mean (SD) minimum spectral power 1340 (10836) v 2616 (1419), p = 0.005) and in patients with non‐sustained ventricular tachycardia (NSVT; n = 7; spectral power 24 h 3427 (1991) v 5223 (2989), p = 0.04; minimum spectral power 1451 (1144) v 2730 (1597), p = 0.03; maximum spectral power 6913 (4497) v 11468 (7388), p = 0.04; LH:HF 2.49 (1.52) v 4.39 (2.4), p = 0.01). We found a correlation between the MWT Z score and SD from the mean of the normal RR intervals at 24 h (p = 0.008; R = 0.36, R2 = 0.12), SD of 5‐min mean RR intervals (p = 0.026; R = 0.30, R2 = 0.09), standard deviations of all normal to normal intervals (p = 0.001; R = 0.49, R2 = 0.24), square root of the mean squared differences of successive RR intervals (p = 0.005; R = 0.37, R2 = 0.14), portion of adjacent RR intervals >50 ms different (p = 0.027; R = 0.30, R2 = 0.09) and maximum spectral power (p = 0.034; R = 0.51, R2 = 0.26), LF (p = 0.007; R = 0.41, R2 = 0.17).
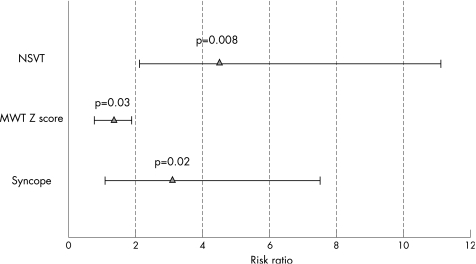
During the follow‐up (mean 5 (2) years), five (9%) patients had a fatal event: two patients had sudden death and three patients had cardiac arrest. Patients with fatal events showed increased mean (SD) MWT Z score (10.2 (3.2) v 6.2 (3.4), p = 0.01), higher prevalence of syncopal events (60% v 12.5%, p = 0.006), ST abnormalities (80% v 31%, p = 0.03), NSVT (60% v 8%, p = 0.001) and increased LF:HF (4.3 (1.7) v 2.6 (1.6), p = 0.04). A logistic multivariate analysis evidenced syncope (p = 0.02; odds ratio (OR) 3.1, 95% confidence interval (CI) 1.4 to 7.6), MWT Z score (OR 1.36, 95% CI 1.02 to 1.89; p = 0.032) and NSVT (OR 4.5, 95% CI 2.1 to 11.3; p = 0.008) as independent predictors of fatal events (fig 1).
Figure 1 Multivariate risk ratio: clinical predictors of major cardiac events. MWT, maximal wall thickness; NSVT, non‐sustained ventricular tachycardia.
Discussion
Sudden death is the most common cause of death in HCM, particularly in children and young people.1,2,3,4,5,6 Several risk markers have been proposed to identify patients with HCM at high risk to die suddenly.1,2,3,4,5 Interestingly, we found an abnormal HRV in patients with known risk markers of sudden death, including syncope, MWT and NSVT. In addition, the increase in the LF:HF ratio in patients with major cardiac events may suggest a correlation between increased sympathetic activity and risk of sudden death in patients with HCM. However, on multivariate logistic regression analysis, syncope, MWT Z score and NSVT were the only independent predictors of adverse outcome in our study population. This is in contrast with previous findings. In a small cohort of children with HCM, Butera et al3 found a reduced LF:HF in those who died suddenly, suggesting a cut‐off LH:HF value of 1.2 as highly predictive of sudden death in their cohort. However, the results of this study are potentially limited by the small number of patients. In addition, the rate of SD in this study is higher than those reported in the literature over the past 10 years.6
In conclusion, our study is important because it failed to confirm a strong association between HRV parameters and a high risk of adverse outcome in HCM. The main clinical implication is the low predictive value of HRV in risk stratification of children and young patients with HCM.
Abbreviations
HCM - hypertrophic cardiomyopathy
HRV - heart rate variability
MWT - maximal ventricular wall thickness
NSVT - non‐sustained ventricular tachycardia
Footnotes
Competing interests: None declared.
References
- 1.McKenna W J, Franklin R C, Nihoyannopoulos P.et al Arrhythmia and prognosis in infants, children and adolescents with hypertrophic cardiomyopathy. J Am Coll Cardiol 198811147–153. [DOI] [PubMed] [Google Scholar]
- 2.Elliott P M, McKenna W J. Hypertrophic cardiomyopathy. Lancet 20043631881–1891. [DOI] [PubMed] [Google Scholar]
- 3.Butera G, Bonnet D, Kachaner J.et al Heart rate variability in children with hypertrophic cardiomyopathy. Heart 200389205–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Counihan P J, Fei L, Bashir Y.et al Assessment of heart rate variability in hypertrophic cardiomyopathy. Association with clinical and prognostic features. Circulation 199388(Pt 1)1682–1690. [DOI] [PubMed] [Google Scholar]
- 5.Yetman A T, Hamilton R M, Benson L N.et al Long‐term outcome and prognostic determinants in children with hypertrophic cardiomyopathy. J Am Coll Cardiol 1998321943–1950. [DOI] [PubMed] [Google Scholar]
- 6.Elliott P M, Gimeno J R, Thaman R.et al Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart 200692785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]