Abstract
Background
Patients with coronary artery disease (CAD) and abnormal glucose regulation (AGR) are at high risk for subsequent cardiovascular events, underlining the importance of accurate glucometabolic assessment in clinical practice.
Objective
To investigate different methods to identify glucose disturbances among patients with acute and stable coronary heart disease.
Methods
Consecutive patients referred to cardiologists were prospectively enrolled at 110 centres in 25 countries (n = 4961). Fasting plasma glucose (FPG) and glycaemia 2 h after a 75‐g glucose load were requested in patients without known glucose abnormalities (n = 3362). Glucose metabolism was classified according to the World Health Organization and American Diabetes Association (ADA; 1997, 2004) criteria as normal, impaired fasting glucose (IFG), impaired glucose tolerance (IGT) or diabetes.
Results
Data on FPG and 2‐h post‐load glycaemia were available for 1867 patients, of whom 870 (47%) had normal glucose regulation, 87 (5%) had IFG, 591 (32%) had IGT and 319 (17%) had diabetes. If classification had been based on the ADA criterion from 1997, the proportion of misclassified (underdiagnosed) patients would have been 39%. The ADA 2004 criterion would have overdiagnosed 8% and underdiagnosed 33% of the patients, resulting in a total misclassification rate of 41%. For ethical concerns and practical reasons, oral glucose tolerance test (OGTT) was not conducted in 1495 of eligible patients. These patients were more often women, had higher age and waist circumference, and were therefore more likely to have AGR than those who were included. A model based on easily available clinical and laboratory variables, including FPG, high‐density lipoprotein cholesterol, age and the logarithm of glycated haemoglobin A1c, misclassified 44% of the patients, of whom 18% were overdiagnosed and 26% were underdiagnosed.
Conclusion
An OGTT is still the most appropriate method for the clinical assessment of glucometabolic status in patients with coronary heart disease.
Abnormal glucose regulation (AGR) has serious prognostic implications in patients with coronary artery disease (CAD). Established diabetes is associated with impaired prognosis after myocardial infarction, and more recent evidence emphasises that the increased risk is already apparent at modestly raised levels of blood glucose below the present threshold for diabetes.1,2 Abnormal glucose metabolism is substantially more common than previously acknowledged both in patients with acute myocardial infarction3 and in those with stable CAD.4 Newly detected abnormal glucose tolerance was recently reported as a strong, independent risk factor for mortality and morbidity after a myocardial infarction.5 Thus, available evidence underlines the importance of appropriate glucometabolic characterisation of patients with CAD.
Substantial discrepancies exist between current recommendations for classification of glucose regulation issued by the World Health Organization (WHO)6 and the American Diabetes Association (ADA).7 ADA criteria are based on fasting plasma glucose (FPG) measurement, whereas both fasting and glycaemia after a 75‐g glucose load are taken into account by the WHO. To increase the likelihood of detecting patients with impaired glucose tolerance (IGT) using FPG, ADA in 2004 lowered the threshold for normal fasting plasma glucose from <6.1 to <5.6 mmol/l.8 The concordance of the new ADA criterion with the WHO categories has so far not been compared in the high‐risk population of patients with CAD.
We tested the hypothesis that an oral glucose tolerance test (OGTT) is still needed for an appropriate classification of glucose regulation in patients with CAD recruited in the Euro Heart Survey on Diabetes and the Heart.4
Methods
Consecutive patients aged >18 years were screened for a diagnosis of CAD when admitted to the hospital cardiology wards or visiting outpatient clinics within several weeks between February 2003 and January 2004 (inclusion time varying between countries) in 110 centres in 25 countries across Europe.4 The survey was an observational study and all patients were assessed, investigated and treated at the discretion of their doctors in charge, according to the usual institutional practice. Among the 4961 patients with confirmed CAD, 1524 (31%) had a prior diagnosis of diabetes, whereas 75 (2%) had previously known impaired fasting glucose (IFG), IGT or were treated with glucose‐lowering drugs (fig 1). The remaining 3362 patients without known glucometabolic abnormalities eligible for evaluation of glycaemic regulation are participants for this study. Data regarding demography, risk factors, medical history, treatment and clinical status at enrolment, together with the reason for consultation, test results and the final diagnosis, were collected for each patient using a web‐based electronic case record form. National requirements for ethical approval were adhered to.
Figure 1 Flow chart of the patient population. CAD, coronary artery disease; CRF, case record form; DM, diabetes mellitus; IGT, impaired glucose tolerance; IFG, impaired fasting glucose; OGTT, oral glucose tolerance test.
Definitions
Coronary artery disease: CAD was diagnosed on clinical grounds supported by at least one objective finding including changes on the electrocardiogram or abnormal stress tests indicating myocardial ischaemia or previous myocardial infarction, or >50% stenosis of lumen diameter of any main coronary artery as seen on a coronary angiogram. Acute myocardial infarction was defined according to the present guidelines.9
Glucose concentration: A measurement of FPG was required for every patient at enrolment or in the morning of the day after hospital admission. The protocol recommended that all patients without previously diagnosed diabetes should undergo a standard OGTT (75 g glucose in 200–250 ml water) in the morning after overnight fast for at least 10 h, according to the WHO recommendations.6 The test was to be performed with the patient in a clinically stable condition, at least 4 days after an acute coronary event, and within 2 months after the index consultation.10 Glucose concentrations were measured according to local routines, if possible in venous plasma, or else in capillary plasma or capillary whole blood. All values are presented as venous plasma glucose using conversion factors established by the European Diabetes Epidemiology Group.11
All glucometabolic classifications according to the WHO (1999)6 and ADA (1997 and 2004)7,8 criteria were based on the measurement of FPG before or 2 h after glucose intake to be reported as normal glucose regulation (NGR), normal fasting glucose, IFG, IGT or diabetes mellitus. Impaired glucose regulation (IGR) encompasses IFG and IGT, whereas AGR comprises IGR and diabetes mellitus. Table 1 outlines the various cut‐off limits.
Table 1 Glucometabolic classification according to the World Health Organization (oral glucose tolerance test) and American Diabetes Association criteria (fasting plasma glucose).
| WHO criteria, 1999 | ADA criteria, 1997 | WHO total (%) | ||
|---|---|---|---|---|
| Normal <6.1 | IFG ⩾6.1 and <7.0 | Diabetes ⩾7.0 | ||
| NGR | 870 (100.0) | 0 | 0 | 870 (46.6) |
| IGR (IGT±IFG) | 494 (72.9) | 184 (27.1)* | 0 | 678 (36.3) |
| Diabetes | 145 (45.4) | 88 (27.6) | 86 (27.0) | 319 (17.1) |
| ADA total (%) | 1509 (80.8) | 272 (14.6) | 86 (4.6) | 1867 (100.0) |
| WHO criteria, 1999 | ADA criteria, 2004 | WHO total (%) | ||
|---|---|---|---|---|
| Normal <5.6 | IFG ⩾5.6 and <7.0 | Diabetes ⩾7.0 | ||
| NGR | 717 (82.4) | 153 (17.6) | 0 | 870 (46.6) |
| IGR (IFG±IGT) | 385 (56.8) | 293 (43.2)† | 0 | 678 (36.3) |
| Diabetes | 93 (29.1) | 140 (43.9) | 86 (27.0) | 319 (17.1) |
| ADA total (%) | 1195 (64.0) | 586 (31.4) | 86 (4.6) | 1867 (100.0) |
ADA, American Diabetes Association; FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IGR, impaired glucose regulation; NFG, normal FPG; NGR, normal glucose regulation; WHO, World Health Organization.
Data are presented as counts and row per cent (%).
*Among those with IFG according to ADA criterion 1997, 87 (32.0%) had IFG only and 97 (35.7%) had IGT and IFG.
†Among those with IFG according to ADA criterion 2004, 109 (18.6%) had IGT only and 97 (16.6%) had IGT and IFG.
Glycated haemoglobin A1c (HbA1c) was determined in capillary blood applied on filter paper12 (Boehringer Mannheim Scandinavian AB, Bromma, Sweden) kept at −20°C until transported to a core laboratory (Department of Clinical Chemistry, Karolinska University Hospital, Solna, Sweden). HbA1c was assayed by high‐performance liquid chromatography (Variant II, Bio‐Rad) with an established method compared with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) standard (r2 = 0.996)13: Swedish HbA1c = 0.989×IFCC HbA1c+0.88%. The upper normal limit is 5.2% and the coefficients of variation are 2.2% and 2.6% for HbA1c at the levels 4.5% and 9.2%, respectively.
Statistical methods
Descriptive statistics were calculated using Statistica V.6.1 (Statsoft). Continuous variables are presented as medians and quartiles and categorical variables as counts and proportions (%). No statistical tests were performed for differences in basic characteristics between the groups. Weighted κ,14 the chance‐corrected proportional agreement, was used to measure the degree of coherence between the OGTT outcome (gold standard) and the outcome of the ADA 1997 and 2004 criteria based on FPG. Data were also classified using ordinal logistic regression: the continuous variables FPG, HbA1c, high‐density lipoprotein cholesterol (HDL‐C), triglycerides, systolic and diastolic blood pressure, body mass index and waist circumference were considered candidate classification variables, together with the dichotomous variables including previous myocardial infarction, stroke, hypertension and family history of diabetes, adjusting for age and sex if necessary. An ordinal logistic regression (Minitab V.13.32, Minitab Ltd, Coventry, UK), assuming proportional odds, was applied for the three classes NGR, IGR and diabetes mellitus.14 All possible combinations of variables were fitted applying 10‐fold cross‐validation, and the models were compared with respect to the proportion of misclassified patients.
Results
Patient characteristics
Table 2 shows the basic characteristics of the 3362 patients. At least one measurement of FPG was reported for 2605 patients and an OGTT was performed in 1867 (56%) patients, of whom 909 (49%) were acutely admitted and 958 (51%) were enrolled following an elective consultation. In 75% of the patients, the OGTT was performed before hospital discharge (92% of the acute and 59% of the elective cohorts), in 94% of patients within 1 month and in all patients within 2 months since recruitment. Those with higher degrees of glucose intolerance were older, had higher waist circumference, FPG, HbA1c and lower HDL‐C and were more often diagnosed with heart failure (table 2).
Table 2 Basic characteristic of patients.
| Parameter | OGTT performed (n = 1867) | No OGTT (n = 1495) | |||
|---|---|---|---|---|---|
| NGR (n = 870) | IGR (n = 678) | DM (n = 319) | Total | ||
| Age (years) | 60 (53–70) | 66 (56–73) | 68 (60–74) | 64 (55–72) | 67 (58–75) |
| Men (%) | 78 | 74 | 75 | 76 | 71 |
| BMI (kg/m2) | 26.8 (24.6–29.4) | 27.5 (25.2–30.1) | 27.5 (25.2–30.3) | 27.1 (24.9–29.8) | 26.7 (24.6–29.3) |
| Waist circumference (cm) | |||||
| Men | 98 (90–104) | 103 (93–106) | 100 (94–107) | 99 (92–105) | 103 (94–103) |
| Women | 94 (84–104) | 95 (87–103) | 98 (88–104) | 95 (86–103) | 98 (86–103) |
| Blood pressure (mm Hg) | |||||
| Systolic | 130 (118–146) | 135 (120–150) | 134 (120–150) | 130 (120–150) | 135 (120–150) |
| Diastolic | 80 (70–90) | 80 (70–90) | 80 (70–90) | 80 (70–90) | 80 (71–90) |
| HbA1c (%) | 4.6 (4.4–4.9) | 4.6 (4.4–5.1) | 4.9 (4.6–5.4) | 4.6 (4.4–5.1) | 4.7 (4.4–5.2) |
| FPG (mmol/l)* | 5.1 (4.7–5.4) | 5.4 (4.9–6.1) | 6.2 (5.4–7.0) | 5.3 (4.80–5.89) | 5.9 (5.3–6.9) |
| Lipids (mmol/l) | |||||
| Total cholesterol | 5.0 (4.3–5.9) | 5.2 (4.4–6.1) | 4.9 (4.2–5.7) | 5.1 (4.3–6.0) | 5.2 (4.4–6.1) |
| HDL‐C | 1.2 (1.0–1.6) | 1.1 (0.9–1.4) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) |
| Triglycerides | 1.5 (1.1–2.1) | 1.6 (1.2–2.1) | 1.6 (1.1–2.0) | 1.5 (1.1–2.1) | 1.6 (1.2,2.1) |
| Medical history (%) | |||||
| MI | 41 | 41 | 39 | 41 | 45 |
| Heart failure | 12 | 16 | 18 | 15 | 21 |
| Hypertension | 56 | 63 | 58 | 59 | 60 |
| Treatment on enrolment (%) | |||||
| β‐Blockers | 52 | 52 | 50 | 51 | 63 |
| ACE or ARB | 45 | 48 | 48 | 47 | 49 |
| Diuretics | 18 | 24 | 23 | 21 | 29 |
| Statins | 45 | 45 | 39 | 44 | 60 |
| Final diagnosis (%) | |||||
| MI Q/non‐Q wave | 32/18 | 12/18 | 37/22 | 34/19 | 25/11 |
| Angina unstable or stable | 18/32 | 19/29 | 19/22 | 19/29 | 26/31 |
| Heart failure | 15 | 20 | 24 | 18 | 25 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; DM, diabetes mellitus; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; IGR, impaired glucose regulation; MI, myocardial infarction; NGR, normal glucose regulation; OGTT, oral glucose tolerance test.
Data are expressed as medians (lower–upper quartiles) unless mentioned otherwise.
*FPB before glucose load when OGTT is performed; in case of no OGTT performed FPG within 24 h since recruitment.
An OGTT was, by various reasons, not performed in 1495 (44%) of the patients. As can be seen in table 2, these patients were somewhat older (67 v 64 years), more often women (29% v 24%) and had a higher waist circumference (men 103 v 99 cm; women 98 v 95 cm) than those who underwent an OGTT.
Glucometabolic classification
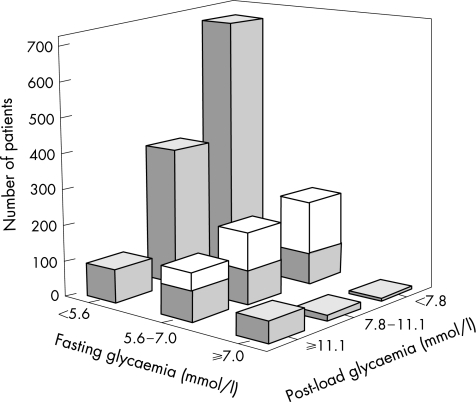
Table 1 details the outcome of glucometabolic classification according to criteria based on OGTT or FPG. Raised FPG concentrations ⩾6.1 or ⩾5.6 mmol/l were found in 19% (n = 358) and 36% (n = 672) of the patients, whereas AGR recognised by OGTT was present in 53% (997) of all participants. The proportion of patients with IGT (n = 591) who had IFG increased from 27% (n = 97) to 35% (n = 206) after the ADA 1997 or ADA 2004 criteria. The agreement expressed as weighted κ was 0.40 (95% confidence interval (CI) 0.36 to 0.44) for ADA 1997 and 0.42 (95% CI 0.38 to 0.46) for ADA 2004 criteria. The proportion of patients misclassified (underdiagnosed) on the basis of the ADA 1997 criterion was 39%. Applying the ADA 2004 criterion, 33% of patients remained underdiagnosed and 8% would have been overdiagnosed, resulting in a total misclassification rate of 41%. Figure 2 gives an overview of the prevalence of different categories of FPG and post‐load glycaemia among patients who underwent an OGTT.
Figure 2 Fasting (FPG) and post‐load plasma glucose in patients with coronary artery disease and no previously known glucose disturbances (n = 1867). Values on the x and z axes represent FPG and 2‐h post‐load venous plasma glucose (mmol/l) taken at the time when oral glucose tolerance test was performed and the patient was in a stable clinical condition. The y axis represents number of patients. The categories of FPG on the x axis correspond to the American Diabetic Association (ADA) 2004 criteria, whereas the categories on the z axis represent the criteria for the 2‐h post‐load glucose according to the World Health Organization (WHO) criteria. The middle bars along the z axis show patients with impaired fasting glucose according to the ADA 2004 criteria divided into two parts: the lower (grey) part represents patients with fasting glycaemia 6.1–7.0 mmol/l (impaired fasting glycaemia according to the WHO or the ADA 1997 classifications). The upper (white) part includes patients with FPG 5.6–6.1 mmol/l, transferred to the category of impaired fasting glucose, when the cut‐off for normal fasting glucose was lowered by ADA from 6.1 to 5.6 mmol/l in 2004.
Estimation of glucose abnormalities
From the set of candidate variables, only FPG, HDL‐C, age and the logarithm of HbA1c had an effect on the glucometabolic categorisation (p<0.20).
The best classification of patients into those with NGR, IGR or diabetes mellitus by applying ordinal logistic regression was achieved by the following equation (table 3; standard error (SE) in brackets): −11.79+1.30×FPG (0.096)+0.035×age (0.006)−0.61×HDL‐C (0.19)+0.81×log (HbA1c) (0.60). A patient was then classified according to the equation (value in brackets) as having NGR (<−2.17), IGR (−2.17 to 0) or diabetes mellitus (>0). The odds of having either IGR or diabetes mellitus increased by 3.70 (95% CI 3.03 to 4.35) for each mmol/l increase in FPG, by 2.22 (95% CI 0.72 to 7.14) with a doubling of HbA1c and by 1.42 (95% CI 1.26 to 1.59) for adding 10 years of age. The odds decreased by 0.54 (95% CI 0.37 to 0.80) for each mmol/l increase in HDL‐C. The weighted κ value was 0.45 (95% CI 0.40 to 0.50)
Table 3 Glucose regulation predicted by logistic regression.
| OGTT | Ordinal logistic regression algorithm | Row total | ||
|---|---|---|---|---|
| NGR | IGR | Diabetes | ||
| NGR | 294 (63.0) | 173 (37.0) | 0 | 467 (47.2) |
| IGR | 142 (40.7) | 198 (56.7) | 9 (2.6) | 349 (35.2) |
| Diabetes | 26 (14.9) | 88 (50.6) | 60 (34.5) | 174 (17.6) |
| Total | 462 (46.7) | 459 (46.3) | 69 (7.0) | 990 (100.0) |
IGR, impaired glucose tolerance; NGR, normal glucose regulation; OGTT, oral glucose tolerance test.
Including fasting plasma glucose, age, high‐density lipoprotein cholesterol and glycated haemoglobin A1c (logistic regression only).
Data are presented as counts and row per cent (%).
This model misclassified 44% of the patients, of whom 18% were overdiagnosed and 26% were underdiagnosed.
Discussion
Our study compared different criteria for the classification of AGR in patients with CAD and without previously known glucometabolic disturbances. The major finding was that an evaluation of glucometabolic status based on fasting glycaemia only, even after applying the most recent ADA criterion, would have misclassified 41% of the patients. Moreover, an algorithm based on easily available clinical and laboratory data was much too imprecise to be of practical value.
Our survey of a large number of people with a wide range of acute or stable CAD gives a good representation of patients seen in all‐day clinical practice. To our knowledge, it represents one of the largest populations of patients with coronary heart disease and no previous glucose disturbances, in whom both FPG and post‐challenge glycaemia were tested.15
Overall prevalence of glucose disturbances
Our data confirm the observation by Norhammar et al3 that previously unknown AGR is common in patients with acute myocardial infarction and extend it, revealing a similar proportion of newly discovered AGR among patients with stable CAD. Studies on the actual prevalence of subclinical hyperglycaemia in patients with CAD rarely include an OGTT. A similar proportion of 18% of cases with unknown diabetes was disclosed by OGTT in patients scheduled for coronary angiography,16 whereas the 19% prevalence of raised FPG (⩾6.1 mmol/l) in our cohort matches the 20% found in the Bezafibrate Infarction Prevention Study.17
The glucose disturbances seem to be twice as common in patients with CAD as in the general population.2,15,18 The frequency of FPG in the diabetic range (⩾7.0 mmol/l) among patients with CAD was not much different from that in the general population in the DECODE Study (5% and 4%), whereas the 2‐h post‐load hyperglycaemia (⩾11.1 mmol/l) was much more common (16% and 4%, respectively; fig 2). The overall prevalence of IFG, as defined by the WHO (⩾6.1 mmol/l) or ADA 2004 (⩾5.6 mmol/l) criteria, was found in 15% and 31% in the present cohort, respectively, which corresponds to reports from the Inter99, Paris Prospective or the NHANES populations. The prevalence of IGT did, however, differ strikingly, with 36% among surveyed patients with CAD compared with 13%, 12% and 8% in the above‐mentioned populations.18
The inter‐relation between IGT and future cardiovascular mortality and morbidity compared with IFG was first shown by the Funagata Diabetes Study.19 Abnormal glucose tolerance was an independent determinant of long‐term outcome in patients treated with coronary angioplasty.20 Recently, the Glucose Tolerance in Patients with Acute Myocardial Infarction Study showed that newly detected abnormal glucose tolerance in patients with an acute myocardial infarction is a strong prognostic predictor during long‐term follow‐up.5 The importance of an early and appropriate recognition of the glucometabolic state in patients with CAD is underlined by its effect on the clinical course and thereby patient management. It is possible to interfere with the decline of glucose homoeostasis and prevent patients with IGT from progressing to diabetes mellitus using lifestyle or pharmacological interventions.21,22 Awareness of the actual glucometabolic state in patients with CAD should contribute to a more aggressive risk factor control.23 That metabolically active treatment modalities may improve the prognosis of patients with cardiovascular disease and AGR is indicated by the Study To Prevent NIDDM24 and the PROspective pioglitAzone Clinical Trial In macroVascular Events trials.25
Diagnostic criteria
The use of both FPG and post‐challenge glycaemia for patient evaluation enabled a comparison of the different classification modalities, with FPG strongly encouraged over an OGTT by ADA.7,8 The degree of disagreement between ADA and WHO criteria was the same whether patients entered the cardiology centres on acute or elective basis. Even if the reproducibility of FPG is higher than that of the 2‐h post‐challenge levels, the reproducibility of recognising newly diagnosed diabetes was virtually identical for the ADA and WHO criteria, 77% and 74%, respectively.26,27 Moreover, weighted κ, expressing the chance‐corrected proportional agreement between the outcome of OGTT and classification not based on postprandial glycaemia, was relatively low.
If only FPG had been used for glucometabolic evaluation, 45% of patients with diabetes shown by an OGTT and 73% with IGT would have remained undiagnosed according to the ADA 1997 criteria.7 Applying the updated ADA criteria,8 these proportions would have been lower, but still substantial, at 29% and 57%, respectively. The overall concordance between IFG (ADA) and IGT (WHO) increased from 5% to 11% by changing the definition of normal FPG from <6.1 to <5.6 mmol/l. Despite this improvement, glucose disturbances would have remained undetected in 21% of all patients with an isolated IGT (fig 2). A new category of patients with FPG concentrations between 5.6 and 6.1 mmol/l, comprising 8% of all patients, would have been classified as prediabetic, creating a potential problem, as their risk of developing diabetes and cardiovascular complications remains unknown. Accordingly, the ADA 2004 criterion evokes concerns by logistic and medical reasons.28,29 Moreover, 2‐h post‐load glycaemia was shown to be a more sensitive predictor for cardiovascular outcomes than FPG.19,29,30 The prognostic potential of the new ADA criterion should therefore be further evaluated before it is generally adopted as an appropriate assessment tool for glucometabolic evaluation of patients with CAD.
Classification without OGTT
Several routine measurements were reported in the survey records, enabling a test of their feasibility for estimation of the glucometabolic state. The best potential predictors were HDL‐C, waist circumference and HbA1c, which were included in the statistical models adjusted for age. Considering a misclassification rate of 44%, the ordinal logistic regression model was not helpful. Thus, an OGTT is still required for appropriate glucometabolic characterisation.
Limitations
The prospective recruitment of patients seen at a large number of European cardiology centres was an advantage, mirroring all‐day practice. Adherence to the volunteer protocol was, however, incomplete, with 1495 eligible patients having not undergone an OGTT and having a somewhat restricted availability of HbA1c samples. The main reason was that ethical permits to perform an OGTT were not issued in some countries. Another but considerably less prevalent reason was technical obstacles experienced in the cardiology care setting for these not‐as‐routine experienced measures. Finally, some patients did not undergo an OGTT because of overt fasting hyperglycaemia that was considered sufficient to establish the diagnosis of diabetes (n = 84; 7.3%). Overall, the patients who did not undergo an OGTT were somewhat older and more often women. They had a higher waist circumference, higher HbA1c values and were more often diagnosed with heart failure (table 2). Any of these features indicate an increased likelihood for having diabetes or impaired glucose regulation. Thus, we can assume that if OGTT would have been tested in all eligible patients, the overall prevalence of AGR would, if anything, be higher than that reported in 1867 patients in whom OGTT was performed. This does not constitute any major concern for the present results, as it is unlikely that diagnostic accuracy would have been different in patients who were not tested.
Ninety six per cent of the population studied was of Caucasian ethnicity. Thus, the results may not apply directly to people of other origins, in particular Asian or Black populations, in whom the prevalence of diabetes is known to be higher.
Conclusions
FPG, regardless of the criteria used to define normality, does not allow a correct recognition of a substantial proportion of people with AGR among patients with CAD. This report will hopefully contribute to a change in clinical practice towards appropriate evaluation of the metabolic state, which presently has to be based on an OGTT, and promote its use so that it will be considered as natural as measuring blood cholesterol. Glucose perturbations carry important prognostic information, and knowledge on this may serve as a platform for improved patient management.
Acknowledgements
We thank Malika Manini for establishing and keeping contact with national coordinators, and Claire Bramley for excellent help in scrutinising the case records and putting the database together.
Abbreviations
ADA - American Diabetes Association
AGR - abnormal glucose regulation
CAD - coronary artery disease
FPG - fasting plasma glucose
HbA1c - glycated haemoglobin A1c
HDL‐C - high‐density lipoprotein cholesterol
IFG - impaired fasting glucose
IGT - impaired glucose tolerance
IGR - impaired glucose regulation
NGR - normal glucose regulation
OGTT - oral glucose tolerance test
WHO - World Health Organization
Footnotes
Funding: This study was supported by grants from the European Society of Cardiology, the Swedish Heart and Lung Foundation, AFA Insurance and King Gustav V and Queen Victoria's Foundation.
Competing interests: None declared.
Contributors: The Euro Heart Survey on Diabetes and the Heart was undertaken under the auspices of the European Society of Cardiology under the initiative of LR who chaired the expert committee on designing and preparation of this survey. Patients were recruited in the respective centres under the supervision of the investigators, whose work was coordinated by national coordinators (as detailed elsewhere).4 Under the supervision of LR and KM, MB prepared proposals of the study protocol and case record forms that were reviewed and approved by all expert committee members. Statistical modelling was conceived and performed by JÖ. Data were analysed by MB and JÖ, who, together with KP, ES, LR and KM, discussed their interpretation. MB, LR and JÖ wrote this report, which was revised by the authors.
References
- 1.Coutinho M, Gerstein H C, Wang Y.et al The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 199922233–240. [DOI] [PubMed] [Google Scholar]
- 2.The DECODE Study Group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetic association diagnostic criteria. Lancet 1999354617–621. [PubMed] [Google Scholar]
- 3.Norhammar A, Tenerz Å, Nilsson G.et al Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 20023592140–2144. [DOI] [PubMed] [Google Scholar]
- 4.Bartnik M, Rydén L, Ferrari R.et al The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. Eur Heart J 2004251880–1890. [DOI] [PubMed] [Google Scholar]
- 5.Bartnik M, Malmberg K, Norhammar A.et al Newly detected abnormal glucose tolerance important predictor of long term outcome after an acute myocardial infarction. Eur Heart J 2004251990–1997. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1 Diagnosis and classification of diabetes mellitus. Geneva: Department of Non‐communicable Disease Surveillance, WHO, 1999
- 7.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997201183–1197. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 200427(Suppl 1)S5–10. [DOI] [PubMed] [Google Scholar]
- 9.Consensus Document Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. Eur Heart J 2000211502–1516. [DOI] [PubMed] [Google Scholar]
- 10.Tenerz A, Norhammar A, Silveira A.et al Diabetes, insulin resistance, and the metabolic syndrome in patients with acute myocardial infarction without previously known diabetes. Diabetes Care 2003262770–2776. [DOI] [PubMed] [Google Scholar]
- 11.The DECODE Study Group on behalf of the European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and non cardiovascular diseases? Diabetes Care 200326688–696. [DOI] [PubMed] [Google Scholar]
- 12.Jeppson J O, Jerntorp P, Almer L O.et al Capillary blood on filter paper for determination of HbA1c by ion exchange chromotography. Diabetes Care 199619142–145. [DOI] [PubMed] [Google Scholar]
- 13.Hoelzel W, Weykamp C, Jeppson J ‐ O.et al IFCC reference system for measurement of hemoglobin‐A1c in human blood and the national standarization schemes in the United States, Japan and Sweden: a method‐comparison study. Clin Chem 200450166–174. [DOI] [PubMed] [Google Scholar]
- 14.Agresti A.An introduction to categorical data analysis. New York: Wiley, 1996
- 15.Wahl P W, Savage P J, Psaty B M.et al Diabetes in older adults. Comparison of 1997 American Diabetes Association classification of diabetes mellitus with 1985 WHO classification. Lancet 19983521012–1015. [DOI] [PubMed] [Google Scholar]
- 16.Taubert G, Winkelmann B R, Schleiffer T.et al Prevalence, predictors, and consequences of unrecognized diabetes mellitus in 3266 patients scheduled for coronary angiography. Am Heart J 2003145285–291. [DOI] [PubMed] [Google Scholar]
- 17.Arcavi L, Behar S, Caspi A.et al High fasting glucose as a predictor of worse clinical outcome in patients with coronary artery disease: results from the Bezafibrate Infarction Prevention Study. Am Heart J 2004147239–245. [DOI] [PubMed] [Google Scholar]
- 18.Borch‐Johnsen K, Colagiuri S, Bakalu B.et al Creating a pandemic of prediabetes: the proposed new diagnostic criteria for impaired fasting glycaemia. Diabetologia 2004471396–1402. [DOI] [PubMed] [Google Scholar]
- 19.Tominaga M, Eguchi H, Manaka H.et al Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 199922920–924. [DOI] [PubMed] [Google Scholar]
- 20.Otsuka Y, Miyazaki S, Okumura H.et al Abnormal glucose tolerance, not small vessel diameter, is a determinant of long‐term prognosis in patients treated with balloon coronary angioplasty. Eur Heart J 2000211790–1796. [DOI] [PubMed] [Google Scholar]
- 21.Tuomilehto J, Lindström J, Eriksson J G.et al Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 20013441343–1350. [DOI] [PubMed] [Google Scholar]
- 22.Knowler W C, Barrett‐Connor E, Fowler S E.et al Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002346393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Backer G, Ambrosioni E, Borch‐Johnsen K.et al Third Joint Task Force of the European and other Societies. European guidelines on CVD prevention. Eur J Cardiovasc Preven Rehabil 200310(Suppl 1)S1–78. [DOI] [PubMed] [Google Scholar]
- 24.Chiasson J L, Josse R G, Gomis R.et al Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP‐NIDDM trial. JAMA 2003290486–494. [DOI] [PubMed] [Google Scholar]
- 25.Dormandy J A, Charbonnel B, Eckland D J.et al Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 20053661279–1289. [DOI] [PubMed] [Google Scholar]
- 26.Ko G T C, Chan J C N, Woo J.et al The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem 19983562–67. [DOI] [PubMed] [Google Scholar]
- 27.deVegt F, Dekker J M, Stehouwer C D A.et al Similar 9‐year mortality risks and reproducibility for the World Health Organisation and American Diabetes Association Glucose Tolerance Categories. Diabetes Care20002340–44. [DOI] [PubMed] [Google Scholar]
- 28.Schringer D L, Lorber B. Lowering the cut point for impaired fasting glucose. Where is the evidence? Where is the logic? Diabetes Care 200427592–595. [DOI] [PubMed] [Google Scholar]
- 29.Barzilay J I, Spiekerman F, Wahl P W.et al Cardiovascular disease in older adults with glucose disorders: comparison of American Diabetes Association criteria for diabetes mellitus with WHO criteria. Lancet 1999354622–625. [DOI] [PubMed] [Google Scholar]
- 30.DECODE Study Group, the European Diabetes Epidemiology Group Glucose tolerance and cardiovascular mortality: comparison of fasting and 2‐hour diagnostic criteria. Arch Intern Med 2001161397–405. [DOI] [PubMed] [Google Scholar]