Prosthetic valve thrombosis (PVT) is a rare but serious complication of valve replacement, most often encountered with mechanical prostheses. The significant morbidity and mortality associated with this condition warrants rapid diagnostic evaluation. However, diagnosis can be challenging, mainly because of variable clinical presentations and the degree of valvular obstruction. Cinefluoroscopy (for mechanical valves) and transthoracic and transoesophageal echocardiography represent the main diagnostic procedures.
Although surgical treatment is usually preferred in cases of obstructive PVT, optimal treatment remains controversial. The different therapeutic modalities available for PVT (heparin treatment, fibrinolysis, surgery) will be largely influenced by the presence of valvular obstruction, by valve location (left‐ or right‐sided), and by clinical status. Hence, treatment of an obstructive left‐sided PVT will differ from that of non‐obstructive or right‐sided PVT. The purpose of this article is to review the physiopathology, diagnosis and treatment of PVT and to provide recommendations for management.
EPIDEMIOLOGY
Mechanical valve thrombosis
The incidence of obstructive PVT for mechanical valves varies between 0.3–1.3% patient years.1 Thromboembolic complications, including systemic emboli, are more frequent and occur at a rate of 0.7–6% patient years. Non‐obstructive PVT is a relatively frequent finding in the postoperative period,2 with a reported incidence as high as 10% in recent transoesophageal echocardiography (TOE) studies. Although these are usually small non‐obstructive thrombi, they underline the necessity of optimal anticoagulation in the postoperative period. According to a series of surgical interventions for PVT, the first postoperative year is marked by a 24% incidence of thrombosis, with a stable incidence between the second to fourth years of approximately 15%, with a subsequent decrease thereafter.3
Bioprosthetic valve thrombosis
Thrombosis of a bioprosthetic valve4 is a rare occurrence when compared to mechanical prostheses. Bioprosthetic PVT is usually diagnosed in the early postoperative period, when endothelialisation of the suture zone is not yet complete. Hence, this has led to the recommendation of anticoagulating patients with bioprostheses for the first three months postoperatively, particularly for mitral prostheses.
PHYSIOPATHOLOGY
Predisposing factors
According to Virchow's triad, factors predisposing to thrombus formation can be divided into endothelial, haemodynamic and haemostatic factors.5 Endothelial factors represent biocompatibility of the prosthesis itself and interaction between the prosthesis and the suture zone. Tissue cicatrisation and endothelialisation characteristically require a few weeks to be complete. Haemodynamic factors include both haemodynamic characteristics of the prosthesis, as well as overall cardiac haemodynamic status. Although the profile of new generation mechanical bileaflet valves is largely superior to that of earlier generation prostheses (and thus associated with a lower occurrence of thromboembolic complications), localised regions of turbulent flow can still develop and lead to stasis and thrombus formation. In addition, the location of the prosthesis plays an important role in thrombogenicity. Obstruction of a tricuspid mechanical prosthesis is 20 times more frequent than left‐sided PVT. Similarly for haemodynamic reasons, mitral PVT is 2–3 times more frequent than thrombosis of an aortic prosthesis. Haemodynamic status can also favour thrombosis, particularly in conditions of low flow or reduced cardiac output. Haemostatic factors involve the adequacy of anticoagulant treatment. In this regard, the early postoperative period represents a particular challenge with the need to balance the risks of over‐anticoagulation and associated haemorrhagic complications with those of under‐anticoagulation and thrombosis.6 Similarly, interruption of oral anticoagulant treatment for anticipated non‐cardiac surgery7 and pregnancy8 represent high risk situations for patients with prosthetic valves.
Pathology
Although PVT can present acutely with a fresh thrombus, it is most often a subacute or chronic phenomenon. Thrombi are typically formed of different clot layers, with variable degrees of organisation. Interestingly, recent surgical studies have underlined the high prevalence of fibrous pannus formation (present between 45–75% of cases), that is also associated with a risk of thrombosis. Caused by an excessive cicatricial response, pannus formation is usually observed in proximity to the suture site and can be located on both sides of the prosthesis, with variable degrees of obstruction.9 Finally, obstruction may also be caused by a vegetation in the context of prosthetic valve endocarditis.
DIAGNOSIS
Clinical presentation
The clinical presentation of PVT is highly variable, often depending on the presence or absence of obstruction. Severe obstructive PVT is typically associated with overt heart failure, whereas non‐obstructive PVT is often an incidental finding or can present as an embolic episode. Partial obstruction (for example, obstruction of one leaflet) can manifest itself with abnormal dyspnoea, or systemic embolism and rarely fever. In the presence of fever, diagnostic blood cultures should be performed to rule out infectious endocarditis. Biological tests typically show normal inflammatory markers, and a subtherapeutic anticoagulation profile. Concentrations of D‐dimers may be raised.
When PVT is first suspected, a careful physical examination should be performed, with particular attention being paid to muffling or disappearance of prosthetic sounds and the appearance of a new regurgitant or obstructive murmur. The initial diagnostic work‐up includes a transthoracic echocardiogram (TTE) and cinefluoroscopy of mechanical valves. TOE will often be performed to complete the investigation. Invasive haemodynamic studies are rarely needed in the evaluation of suspected PVT.
Cinefluoroscopy
As all commercially available mechanical valves available since the 1980s are radio‐opaque, cinefluoroscopy (fig 1) is an important part of the diagnostic evaluation of suspected PVT. To increase diagnostic accuracy, several procedures should be performed to visualise leaflet mobility optimally. However, this technique will not be helpful in identifying non‐obstructive PVT or differentiating pannus from thrombus. Hence, additional diagnostic procedures are often necessary.10
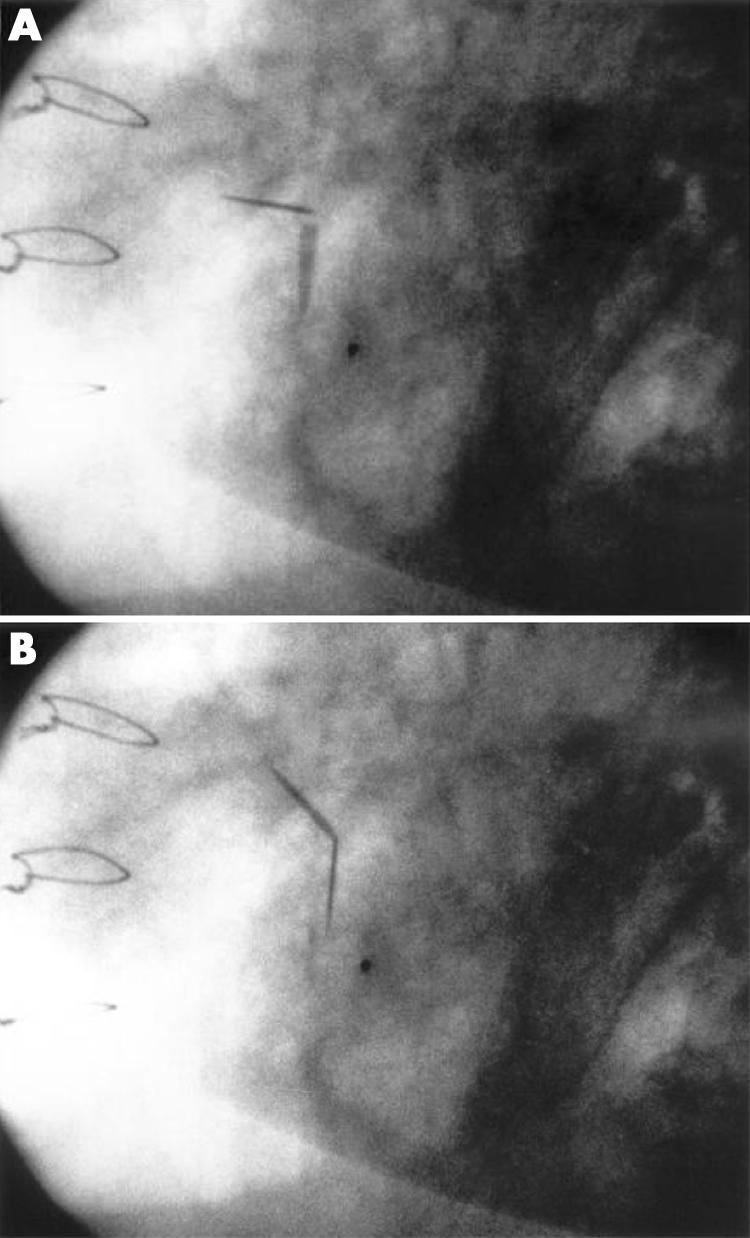
Figure 1 Cinefluoroscopy showing impaired motion of the two leaflets of a mechanical mitral valve.
Transthoracic echocardiography
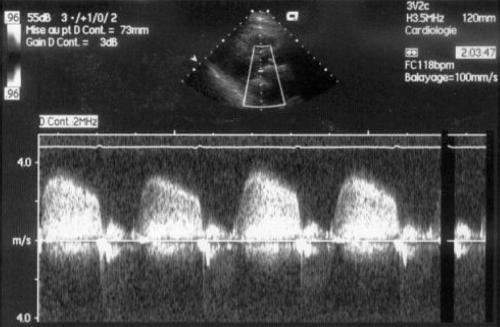
By providing direct visualisation of the prosthesis and a measure of transvalvular gradients, TTE is an essential part of diagnostic assessment (fig 2, table 1). A standard complete examination should be performed, with particular attention directed at transvalvular flow, gradient, and inspection of the prosthesis.11
Figure 2 Transthoracic echocardiography in a patient with a thrombosed bileaflet mitral valve; mean gradient was 30 mm Hg.
Table 1 Echocardiographic signs of obstructive prosthetic valve thrombosis.
| • Reduced valve mobility |
| • Presence of thrombus |
| • Abnormal transprosthetic flow |
| • Central prosthetic regurgitation |
| • Elevated transprosthetic gradients |
| • Reduced effective prosthetic area |
Using colour Doppler, abnormal transprosthetic flow (as indicated by aliasing) or central regurgitation, indicating abnormal valve closure, can be observed. Transprosthetic gradients and effective orifice area are determined using continuous Doppler. Pulmonary artery pressures and cardiac output should also be measured. Direct signs of PVT include abnormal movement of the prosthesis (immobile hemi‐disc, incomplete or delayed opening) or visualisation of a paraprosthetic thrombus.
For mitral prostheses, a mean gradient >8 mm Hg and an effective area ⩽1.3 cm2 is indicative of PVT (fig 1). For aortic prostheses, criteria for PVT are a mean gradient >45 mm Hg and an obstructive index <0.25. In the case of small size aortic prostheses (for example, mechanical valve sizes 19 or 21), diagnosis can be difficult when considering that these prostheses often have “normally” elevated gradients, due to local turbulent flow across the main orifice and potential patient–prosthesis mismatch.12 In these patients, the following criteria will argue against a diagnosis of PVT: an already elevated gradient on previous echocardiographic examinations, an obstructive index >0.25, an effective orifice area >0.7 cm2, valvular resistance <280 dynes.s.cm−5, and normal leaflet mobility on cinefluoroscopy.13
Other Doppler indexes have been proposed to detect prosthetic mitral valve dysfunction. In a study of 134 patients with mitral prostheses, Fernandes et al identified 95% of patients with valve obstruction using the following criteria: peak E velocity ⩾1.9 m/s, VTImitral/VTIaortic ⩾2.2, and pressure half‐time >130 ms.14
Proper interpretation of transvalvular gradients and valve areas requires comparison with standardised reference tables, available for each prosthesis type and size. It is also important to compare results with the patients' individual reference values, ideally obtained within the first postoperative month after a period of haemodynamic stabilisation.
Limitations of TTE include quality of the acoustic window, artefacts associated with the prosthesis, and non‐obstructive PVT (where TTE will usually be normal). In conditions of low cardiac output, transvalvular gradients can be within the normal range despite significant prosthetic valve obstruction, so‐called “silent Doppler PVT”. Hence if clinical suspicion remains, the investigation should be completed with a TOE study.
Transoesophageal echocardiography
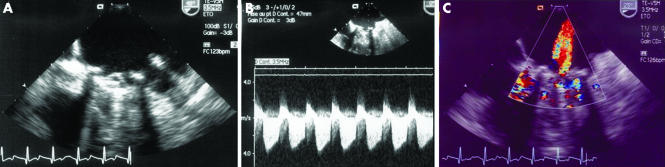
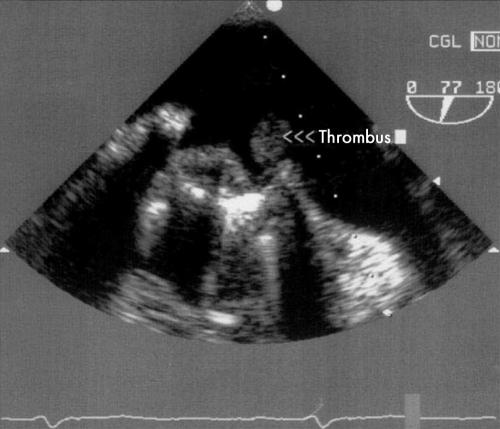
In the rare case of massive PVT with haemodynamic instability, TOE will not be needed, as this dramatic presentation constitutes a surgical emergency. In most other cases, the high resolution imaging of TOE (figs 3 and 4) will provide important additional diagnostic information, which will also guide treatment.15,16
Figure 3 Transoesophageal echocardiography in the same patient shown in fig 2. (A) Stuck leaflet, (B) small thrombus, and (C) central mitral regurgitation.
Figure 4 Transoesophageal echocardiography: stuck mitral valve and pedunculated thrombus.
Direct signs of PVT include immobility or reduced leaflet mobility, and the presence of thrombus on either side of the prosthesis, with or without obstruction. Thrombi have to be differentiated from a fibrous pannus, which is usually annular in location. Pannus formation is more frequent on aortic than on mitral prostheses. When observed on mitral prosthetic valves, they most often occur on the atrial side of the prosthesis. Typically presenting as a very dense immobile echo, pannus are typically encountered in patients with a normal anticoagulation profile and with subacute or chronic symptoms. Indirect TOE signs of PVT are the disappearance of the normal physiological prosthesis regurgitant flow, the presence of central prosthesis regurgitation, and pronounced spontaneous echo contrasts in the left atrium.
As with any other diagnostic modality, TOE has some limitations: aortic prostheses are more difficult to evaluate than mitral prostheses, and the ventricular side of a mitral prosthesis is more difficult to evaluate than the atrial side. It is also important to differentiate small thrombi from strands or sutures. Strands are believed to be fibrin filaments, appearing as fine (1 mm), filamentous, mobile echos of variable length (around 10 mm), most often observed on the atrial side of mitral prostheses. Although the precise nature of these strands remains undetermined, recent studies do not suggest an embolic risk.
Finally, one must underline the important role of TOE in guiding therapeutic strategy. For left‐sided obstructive PVT, the presence of a large thrombus favours surgery, as fibrinolysis carries a significant embolic risk. The international PRO‐TEE registry identified a previous cerebrovascular event and a thrombus size >0.8 cm2 as the major risk factors for complications of lytic treatment.17 In cases of non‐obstructive PVT, the preferred treatment is usually medical therapy, unless the thrombus is large or highly mobile. TOE is also helpful in differentiating a pannus from a thrombus, and in determining thrombus size accurately.
Thrombosis of a bioprosthesis has a similar clinical presentation to that of mechanical valve PVT. In cases of obstruction, TTE will demonstrate abnormal transvalvular gradients and exceptionally the presence of a thrombus. TOE has a much greater sensitivity than TTE for the diagnosis of bioprosthesis thrombosis.
Other techniques
Recently, a new device has been introduced to allow home monitoring of heart valve function. The hand‐held Thrombocheck device is designed to detect subtle changes in acoustic sounds of prosthetic valves and thus identify early valve dysfunction. A preliminary study in 71 patients with one or more bileaflet mechanical valves allowed detection of prosthetic valve dysfunction with a sensitivity of 90% and a specificity of 98%. Although these early results will need confirmation, this technique could be useful as routine non‐invasive monitoring of prosthetic valves. Multidetector row computed tomography is another potential new technique that could help to identify pannus formation.
TREATMENT
Once diagnosis of PVT is confirmed, several therapeutic modalities can be considered: surgery, fibrinolysis, heparin treatment, or optimisation of anticoagulant and antiplatelet therapy. Treatment can be separated according to presence of obstruction and prosthesis location. The type of prosthesis (mechanical or biological) does not have particular therapeutic implications, as the choice between surgery and medical treatment will have to take into account the same considerations, namely prosthesis location, thrombus size and clinical status.
Non‐obstructive left‐sided PVT
The introduction of routine TOE has allowed us to better define the optimal therapeutic strategy for non‐obstructive PVT.2,6,18 Management can be divided according to thrombus size:
For large (>5 mm) non‐obstructive thrombi, surgery may be indicated in cases of failure of medical treatment (heparin), particularly in the presence of large, mobile and pedunculated thrombi. However, case reports have demonstrated the disappearance of mobile, fresh thrombi with heparin treatment alone in patients with subtherapeutic anticoagulation.
For small (<5 mm) non‐obstructive thrombi, medical treatment is preferred.
Treatment can comprise heparin therapy for one week with repeat TOE to evaluate treatment efficacy, or adjustment of warfarin therapy with addition of low dose aspirin (100 mg). Fibrinolysis has been performed with success for small non‐obstructive thrombi, but with a significant risk of systemic embolism. In all cases of PVT, anticoagulant treatment has to be optimally adjusted.
Obstructive PVT
Obstruction of a mechanical prosthesis requires aggressive treatment (surgery or fibrinolysis), as anticoagulant treatment will usually be insufficient. Because of the lack of randomised studies, there are few recommendations in the literature concerning PVT management (level 2 recommendations when available). According to the American College of Cardiology/American Heart Association guidelines,19 surgery is the preferred treatment for left‐sided PVT. Fibrinolysis should be reserved for patients with poor functional class (New York Heart Association (NYHA) III or IV), with high surgical risk or contraindications to surgery. It can also be considered in patients with good functional class (NYHA I or II) and a small thrombus, after failure of heparin treatment.
Right‐sided obstructive PVT
Mechanical prosthetic valves are rarely implanted in the right heart, mainly because of their important thrombogenicity. Obstruction of a tricuspid or pulmonary prosthesis is usually considered an indication for fibrinolysis.20 Some studies have shown the therapeutic benefit of fibrinolysis in right‐sided PVT with an acceptable complication rate. This efficacy usually obviates the need for surgery, which will be reserved for cases of fibrinolysis failure.
Left‐sided obstructive PVT
On the contrary, obstructive left‐sided PVT is considered an indication for surgery, with fibrinolysis reserved for particular situations.21,22,23 Historically considered as very high risk surgery (with reported operative mortality of patients with NYHA class IV symptoms of around 50%), recent advances in surgery, anaesthesia and perioperative care have significantly improved prognosis. According to a recent series, mortality was 4% for patients with NYHA class I, II and III, whereas it reached 17.5% in patients with NYHA class IV symptoms. The intervention can involve valvular replacement (with bioprosthesis implantation in cases of recurrent thrombosis) or simple thrombectomy.
Fibrinolysis has been introduced as a therapeutic alternative in the 1970s. A recent review published by Lengyel et al24 found fibrinolysis to be efficacious in 82% of cases, but with an associated 10% mortality rate and a 12.5% rate of systemic emboli. This high complication rate has relegated fibrinolysis to second line therapy, reserved mainly for patients with contraindications to surgery such as advanced age, severe left ventricular dysfunction or multiple previous operations.
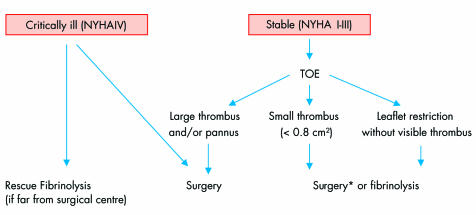
A simple algorithm (fig 5) for the management of left‐sided obstructive PVT can be proposed and should take into account several factors: clinical status (NYHA functional class, acuteness of presentation), contraindications to surgery, thrombus size, distance from the operating room and, in some countries, economic factors.
Figure 5 Management of patients with obstructive left‐sided prosthetic valve thrombosis. *Surgery preferred for first generation prosthesis. Fibrinolysis may be considered first‐line treatment in patients with poor general medical condition or contraindications to surgery. NYHA, New York Heart Association; TOE, transoesophageal echocardiography.
Fibrinolytic treatment protocols
There is no consensus in the literature concerning the best fibrinolytic regimen. A simple therapeutic strategy can be proposed with two types of protocols. In patients with haemodynamic instability, “rescue” fibrinolysis should be preferred, using a “short protocol” consisting of either:
Prosthetic heart valve thrombosis: key points
Prosthetic valve thrombosis (PVT) is a serious complication of valvular replacement associated with significant morbidity and mortality
The incidence is higher for mechanical than for biological heart valves (right‐sided > left‐sided, mitral > aortic). Non‐obstructive thrombi are more frequently observed than obstructive thrombi
The early postoperative period, interruption of anticoagulant therapy for non‐cardiac surgery, and pregnancy are particularly high‐risk situations for patients with prosthetic heart valves
Diagnosis is based on cinefluoroscopy (for mechanical valves) and echocardiography. Suggestive findings include reduced or absent leaflet mobility, elevated transprosthetic gradients, decreased effective orifice area and thrombus visualisation
Transoesophageal echocardiography (TOE) has an important diagnostic role, particularly in difficult cases, and can differentiate thrombi from pannus formation or strands. TOE is also important in guiding treatment
Treatment of left‐sided PVT is influenced by clinical status, thrombus size and presence of obstruction. Mobile or large thrombi (>0.8 cm2) are usually treated surgically. Critically ill patients (NYHA functional class IV), obstructive PVT and large non‐obstructive PVT are indications for surgery. Obstructive PVT with a small thrombus and stable clinical condition (NYHA classes I–III) can be treated by either surgery or fibrinolysis. Non‐obstructive PVT with a small thrombus (<5 mm) can be treated by fibrinolytic therapy
Fibrinolysis has a good success rate in the treatment of right‐sided PVT, with a low rate of associated complications
recombinant tissue plasminogen activator (rtPA) 10 mg bolus + 90 mg in 90 mins, or
streptokinase 1 500 000 U in 60 mins without heparin.
In haemodynamically stable patients, a long protocol is often preferred using either:
urokinase 4500 U/kg/h over a 12 h period, or 2000 U/kg/h + heparin over 24 h, streptokinase 500 000 IU in 20 mins followed by 1 500 000 IU for 10 h without heparin
rtPA 10 mg bolus, 50 mg during the first hour, 20 mg during the second hour and 20 mg during the third hour.
The combination of fibrinolytic agents has also been described, particularly for incomplete responses to a first agent. The major risk of fibrinolytic treatment is systemic emboli, with or without associated sequelae.
As already mentioned, the role of diagnostic techniques in defining the optimal treatment modality is primordial. Montorsi et al have shown the presence of a completely immobile leaflet at fluoroscopy, in patients with symptoms present for more than three weeks, to be associated with more organised lesions, usually requiring surgical intervention. In our experience, TOE has an essential role in patient selection for eventual lytic treatment, as a large thrombus is associated with a significantly greater embolic risk. Fibrinolysis has good efficacy for small thrombi which have appeared recently.
CONCLUSION
The mortality associated with obstructive prosthetic valve thrombosis is approximately 10%, independent of treatment modality. Thus, suspicion of PVT is an urgent clinical condition, which warrants rapid diagnostic assessment. Diagnosis will be based on the findings of the clinical examination, cinefluoroscopy and echocardiography. The role of TOE is fundamental, not only for accurate diagnosis but also to provide optimal treatment. Therapeutic strategy will be influenced by prosthesis location, the presence or absence of valvular obstruction, and by the patients' clinical status.
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Supplementary Material
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
References
- 1.Horstkotte D, Burckardt D. Prosthetic valve thrombosis. J Heart Valve Dis 19954141–153. [PubMed] [Google Scholar]
- 2.Laplace G, Lafitte S, Labèque J N.et al Clinical significance of early thrombosis after prosthetic mitral valve replacement. J Am Coll Cardiol 2004431283–1290.A postoperative monocentric study of 680 patients which finds 9.4% of thrombi are essentially non‐obstructive. [DOI] [PubMed] [Google Scholar]
- 3.Deviri E, Sareli P, Wisenbaugh T.et al Obstruction of mechanical heart valve prostheses: clinical aspect and surgical management. J Am Coll Cardiol 199117646–650.An important series analysing surgical findings in 100 patients operated for PVT, and underlying the role of pannus formation. [DOI] [PubMed] [Google Scholar]
- 4.Heras M, Chesebro J H, Fuster V.et al High risk of thromboemboli early after bioprosthetic cardiac valve replacement. J Am Coll Cardiol 1995251111–1119. [DOI] [PubMed] [Google Scholar]
- 5.Gencbay M, Turan F, Degertekin M.et al High prevalence of hypercoagulable states in patient with recurrent thrombosis of mechanical heart valves. J Heart Valve Dis 19987601–609. [PubMed] [Google Scholar]
- 6.Iung B, Cormier B, Dadez E.et al Small abnormal echoes after mitral valve replacement with bileaflet mechanical prostheses: predisposing factors and effect on thromboembolism. J Heart Valve Dis 19932259–266. [PubMed] [Google Scholar]
- 7.Gohlke‐Bärwolf C. Anticoagulation in valvar heart disease: new aspects and management during non‐cardiac surgery. Heart 200084567–572.This is a thorough review of clinically important topics for anticoagulation in patient management. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahnoun‐Trabelsi I, Jimenez M, Choussat A.et al Thromboses de prothèses valvulaires cardiaques chez la femme enceinte. Arch Mal Cœur 200497305–310. [PubMed] [Google Scholar]
- 9.Barbetseas J, Nagueh S F, Pitsavos C.et al Differentiating thrombus from pannus formation in obstructed mechanical prosthetic valves: an evaluation of clinical transthoracic and TEE parameters. J Am Coll Cardiol 1998321410–1417. [DOI] [PubMed] [Google Scholar]
- 10.Montorsi P, De Bernardi F, Muratori M.et al Role of cine‐fluoroscopy, transthoracic, and transesophageal echocardiography in patients with suspected prosthetic heart valve thrombosis. Am J Cardiol 20008558–64.The aim of this work was to evaluate the diagnostic efficacy of the three methods in 82 consecutive patients with suspected PVT. [DOI] [PubMed] [Google Scholar]
- 11.Habib G, Cornen A, Mesana T.et al Diagnosis of prosthetic heart valve thrombosis, the respective value of transthoracic and transoesophageal echocardiography. Eur Heart J 199314447–455. [DOI] [PubMed] [Google Scholar]
- 12.Pibarot P, Dumesnil J G. Hemodynamic and clinical impact of prosthesis‐patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol 2000361131–1141. [DOI] [PubMed] [Google Scholar]
- 13.Saad R M, Barbetseas J, Olmos L.et al Application of the continuity equation and valve resistance to the evaluation of St Jude Medical prosthetic aortic valve dysfunction. Am J Cardiol 1997801239–1242. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes V, Olmos L, Nagueh S F.et al Peak early diastolic velocity rather than pressure half‐time is the best index of mechanical prosthetic mitral valve function. Am J Cardiol 200289704–710.The purpose of this study was to test the accuracy of Doppler parameters in identifying patients with PVT. [DOI] [PubMed] [Google Scholar]
- 15.Dzavik V, Cohen G, Leung Chan K. Role of transesophageal echocardiography in the diagnosis and management of prosthetic valve thrombosis. J Am Coll Cardiol 1991181829–1833. [DOI] [PubMed] [Google Scholar]
- 16.Guéret P, Vignon P, Fournier P.et al Transesophageal echocardiography for the diagnosis and management of non obstructive thrombosis of mechanical mitral valve prosthesis. Circulation 199591103–110.This study confirms the leading role of TOE in the diagnosis of non‐obstructive PVT. [DOI] [PubMed] [Google Scholar]
- 17.Tong A T, Roudaut R, Ozkan M.et al Transesophageal echocardiography improves risk assessment of thrombolysis of prosthetic valve thrombosis: results of the international PRO‐TEE registry. J Am Coll Cardiol 20044377–84.This multicentre study of more than 100 patients underlines the role of large thrombi and history of stroke as risk factors for complication of fibrinolysis. [DOI] [PubMed] [Google Scholar]
- 18.Laffort P, Roudaut R, Roques X.et al Early and long‐term (one‐year) effects of the association of aspirin and oral anticoagulant on thrombi and morbidity after replacement of the mitral valve with the St. Jude medical prosthesis. J Am Coll Cardiol 200035739–746. [DOI] [PubMed] [Google Scholar]
- 19.Bonow R O, Carabello B, De Lean A C., Jret al ACC/AHA guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 1998981949–1984. [DOI] [PubMed] [Google Scholar]
- 20.Shapira Y, Sagie A, Jortner R.et al Thrombosis of bileaflet tricuspid valve prosthesis: clinical spectrum and the role of nonsurgical treatment. Am Heart J 1999137721–725. [DOI] [PubMed] [Google Scholar]
- 21.Roudaut R, Lafitte S, Roudaut M F.et al Fibrinolysis of mechanical prosthetic valve thrombosis: a single‐center study of 127 cases. J Am Coll Cardiol 200341653–658.This single centre series underlines the risk of embolism during fibrinolysis, with possible permanent damage. [DOI] [PubMed] [Google Scholar]
- 22.Roudaut R, Roques X, Lafitte S.et al Surgery for prosthetic valve obstruction. A single center study of 136 patients. Eur J Cardiothorac Surg 200324868–872.This monocentric study points to the improvement of surgical results in recent years. [DOI] [PubMed] [Google Scholar]
- 23.Alpert J S. The thrombosed prosthetic valve. Current recommendations based on evidence from the literature. J Am Coll Cardiol 200341659–660. [DOI] [PubMed] [Google Scholar]
- 24.Lengyel M, Fuster V, Keltai M.et al Guidelines for management of left sided prosthetic valve thrombosis: a role for thrombolytic therapy. J Am Coll Cardiol 1997301521–1526.This international conference led to the development of guidelines for the management of left‐sided PVT. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.