Coronary artery disease (CAD) remains the leading cause of death in the United States and Europe. Although there has been a decline in the number of deaths from myocardial infarction throughout the western world, the mortality for congestive heart failure has more than doubled. Importantly, CAD accounts for the majority (almost 70%) of congestive heart failure cases.
In the clinical management of patients with congestive heart failure caused by CAD, the accurate assessment of myocardial viability is crucial to guide treatment; this is because revascularisation of dysfunctional but viable myocardium can improve ventricular function and long‐term survival.
Another everyday clinical scenario is the risk stratification of patients suspected to have CAD presenting for work up of chest pain syndromes. If left ventricular function is normal, stress testing remains the primary approach for detecting myocardial ischaemia.
Traditionally nuclear imaging, stress echocardiography and (stress) electrocardiography have been the clinical mainstays for assessing myocardial viability as well as to detect myocardial ischaemia. However, cardiovascular MR (CMR) is a rapidly emerging non‐invasive imaging technique, providing high‐resolution images of the heart in any desired plane and without radiation. Rather than a single technique, CMR consists of several techniques that can be performed separately or in various combinations during a patient examination. For example, cine‐CMR can provide assessment of cardiac morphology, function and contractile reserve; perfusion CMR with and without vasodilators can provide assessment of myocardial perfusion reserve, and contrast enhanced CMR (ceCMR) can be used for infarct detection as well as non‐invasive tissue characterisation.
This article will review the potential of CMR for managing patients with known or suspected CAD by discussing different CMR techniques for assessment of myocardial viability and myocardial ischaemia.
ASSESSMENT OF MYOCARDIAL VIABILITY
The most precise definition of infarction, and therefore the loss of viability, is that myocyte death must have occurred. All ischaemic events before cell death are, at least in principle, reversible by re‐establishment of an adequate blood supply. The presence or absence of cell death can be established by light microscopy, electron microscopy, or by the use of histologic stains such as triphenyl tetrazolium chloride (TTC). However, testing for myocardial viability by microscopy or histologic staining is obviously not practical in a clinical setting. Consequently, a number of less precise definitions of viability have been developed, which are based on parameters more easily measured in patients:
(less precise)
wall motion abnormality↓
Q waves, total enzyme leak↓
no‐reflow or low re‐flow↓
change in tissue composition↓
myocyte integrity
(more precise)
It is important to recognise, however, that these clinical definitions are indirect and that only demonstration of the presence of living myocytes can be considered to be the ultimate proof of the presence of viable myocardium.
DOBUTAMINE CMR
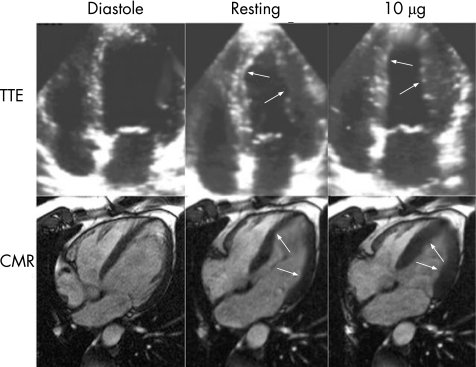
Contractile reserve can be assessed by CMR using low dose dobutamine stress testing. Similar to echocardiography, CMR allows visualisation of cardiac motion but is characterised by superior endocardial border definition, facilitating more accurate wall motion and wall thickening assessment (fig 1).
Figure 1 Direct comparison of a low dose dobutamine transthoracic echo (TTE) and low dose dobutamine cardiovascular magnetic resonance (CMR) study to assess viability in the septal and lateral wall after myocardial infarction in the same patient. Both techniques are capable of visualising an improvement of wall motion after dobutamine infusion (see arrows) as a sign of residual viability in the areas of interest. However, CMR images are characterised by superior endocardial border definition, facilitating more accurate wall motion assessment, even in this patient with good acoustic window. This advantage of CMR is especially important in patients with poor acoustic windows. Adapted with permission from Mahrholdt et al. In: Picano E, ed. Stress echocardiography versus magnetic resonance imaging. Berlin: Springer, 403–18.
There is a large body of evidence that an increase in regional systolic function with low dose dobutamine is indicative of myocardial viability. Dobutamine CMR compared favourably to positron emission tomography (PET) in 35 patients with mild left ventricular dysfunction, with a sensitivity of 88% and a specificity of 87% for detecting regions of viable myocardium.1 When the recovery of regional improvement of wall thickening after revascularisation was considered to be the gold standard, the majority of studies showed a relatively modest sensitivity but high specificity of dobutamine CMR for detection of viable myocardium, ranging from 50–90% and 73–94%, respectively. Analogous to the limitations of other viability tests, the reported accuracy of CMR depends on the studied population, since it is known that contractile reserve has a reduced predictive accuracy if more severe dysfunction is present at rest. Whereas Baer et al1 found a good sensitivity and specificity in patients with mild left ventricular (LV) dysfunction (mean (SD) ejection fraction was 42 (16)%), Gunning et al,2 who studied 30 patients with more severe LV dysfunction (mean ejection fraction 24 (8)%), confirmed the high specificity of 81% but found a sensitivity of only 50%. This reduced sensitivity may relate to the development of ischaemia at even low levels of inotropic stress, and/or the pathophysiology of hibernating myocardium in which cellular dedifferentiation may occur with dropout of myofibrillar units. In both cases, viable myocardium would be unable to respond to inotropic stimulation. Consequently, if contractile function improves after inotropic stimulation, it is safe to assume that there is a significant amount of viability; the converse, however, is not necessarily true.
CONTRAST ENHANCEMENT TECHNIQUE
Contrast enhanced CMR is a newly established technique for infarct assessment. Regions of myocardial infarction exhibit high signal intensity (contrast enhancement) on T1‐weighted images after administration of extracellular CMR imaging contrast such as gadolinium based agents.
The most likely mechanism of contrast enhancement in infarction is that in acutely infarcted regions extracellular contrast agent passively diffuses into the intracellular space due to rupture of myocyte membranes ( = myocyte death), resulting in an increased tissue‐level contrast concentration and therefore contrast enhancement. Chronic infarcts are characterised by collagenous scar ( = absence of living myocytes) with increased interstitial space between collagen fibres, also resulting in an increased contrast concentration and thus contrast enhancement.
With the recent introduction of an optimised segmented inversion recovery gradient recalled echo sequence, infarcted myocardium can be visualised with substantially improved contrast and image quality compared to traditional spin echo images.3 Contrast images are acquired in mid‐diastole by using a trigger delay to minimise the cardiac motion. The magnetisation of the heart is prepared by a non‐selective 180*‐inversion pulse to increase T1‐weighting. For correct implementation the inversion time (delay between inversion pulse and data collection) must be manually selected to null signal from normal myocardial regions.4 The inversion time needed to null signal from normal myocardium varies from patient to patient as a function of dose and also varies with time after contrast administration due to contrast agent pharmacokinetics.4
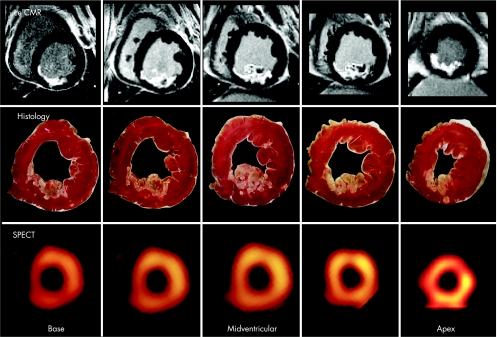
Contrast enhanced CMR using this technique has been extensively validated in animal models of ischaemic injury and a variety of patient cohorts. Animal studies proved that ceCMR can differentiate between viable and non‐viable myocardium regardless of wall motion abnormality at rest, the age of the infarction, or the perfusion status with a near perfect correlation between enhancement on ceCMR and irreversible damage at pathology (upper two rows in fig 2).5 Additionally, numerous patient studies have confirmed that ceCMR is effective in identifying the presence, location and extent of acute and chronic myocardial infarction.4,5
Figure 2 Contrast enhanced cardiovascular magnetic resonance (CeCMR), histology and single photon emission computed tomography (SPECT) images obtained in an animal with a medium sized infarct. There is a nearly perfect match between necrosis defined by histology and ceCMR. Whereas ceCMR allows the exact assessment of the transmural extent of infarction, SPECT defines segments as either viable or non‐viable. Reproduced with permission from Wagner et al.9
The comparison of ceCMR with other modalities for the discrimination of viable and non‐viable myocardium has been favourable. Ansari et al6 reported a strong correlation between segments defined as infarcted by ceCMR and defined as non‐viable by thallium rest redistribution single photon emission computed tomography (SPECT) (<50% of maximal thallium uptake) in patients with prior myocardial infarction and left ventricular dysfunction, with an inverse relationship between the area of contrast enhancement and diminished thallium uptake. Klein et al7 found that the area of contrast enhancement measured by ceCMR correlated closely with myocardial infarcts defined by PET in patients with ischaemic cardiomyopathy. Kühl et al8 compared F‐18 fluorodeoxyglucose (FDG) PET and ceCMR in 26 patients. The study showed that segmental glucose uptake by PET inversely correlated with the segmental extent of contrast enhancement, and that using a cut off value of 37% segmental enhancement optimally differentiated viable from non‐viable segments defined by PET; using PET as the gold standard, this resulted in a sensitivity and specificity of ceCMR for detection of viable myocardium of 96% and 84%, respectively.
Because of its superior spatial resolution, CMR has the unique ability to assess small infarcts and to measure the transmural extent of myocardial infarction. This advantage has been used to detect micro‐infarcts associated with successful coronary angioplasty, as well as the detection of subendocardial infarcts which are missed by SPECT9 (fig 2) or do not exhibit a wall motion abnormality.10
The ability to assess accurately the transmural extent of infarction has laid the foundation for subsequent investigations in animals and humans demonstrating that the extent of contrast enhancement on a segmental or regional basis is useful for the prediction of improvement in contractile function after revascularisation in CAD patients.
Hillenbrand et al11 were the first to demonstrate in an animal model that the transmural extent of contrast enhancement predicts myocardial salvage after acute myocardial infarction. Similar results were found in patients. Choi et al12 scanned 24 patients within seven days after acute myocardial infarction and successful revascularisation. As in the animal data, the improvement in contractile function was inversely correlated to the degree of transmural involvement of the infarction.
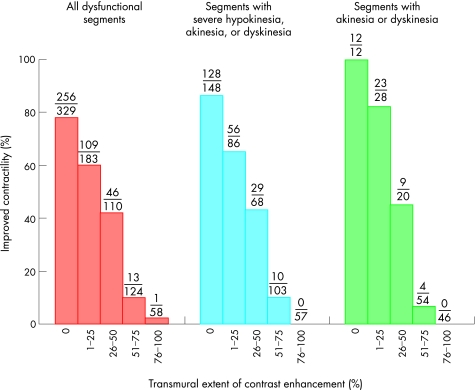
Additionally it has been shown that ceCMR is able to predict functional recovery in patients with chronic ischaemia. Kim et al13 performed the initial assessment of ceCMR in this patient cohort. Fifty consecutive patients with chronic heart failure were scanned before they underwent revascularisation. When the volume of dysfunctional but viable myocardium before revascularisation was calculated on a patient‐by‐patient basis, an increasing extent of dysfunctional but viable myocardium correlated with greater improvements in both the mean wall motion score and the ejection fraction after revascularisation. Considering all dysfunctional segments before revascularisation, the proportion with contractile improvement decreased progressively as the transmural extent of contrast enhancement increased (fig 3). For example, 256 of 329 segments (78%) without any infarction, but only one of 58 segments with more than 75% transmural extent of infarction on the initial scan, improved at follow up approximately 11 weeks later. The likelihood of functional improvement in regions without contrast enhancement was 86% for segments with at least severe hypokinesia, and 100% for segments with akinesia or dyskinesia. Thus, unlike dobutamine echo and dobutamine CMR, which appear to have reduced predictive accuracy if more severe dysfunction is present, ceCMR seems to have greater accuracy in segments with most severe dysfunction.
Figure 3 Relationship between the transmural extent of contrast enhancement before revascularisation and the likelihood of increased contractility after revascularisation. Reproduced with permission from Kim et al.13
Myocardial viability: key points
The diagnostic performance of dobutamine cardiovascular magnetic resonance imaging (CMR) is at least comparable to dobutamine echocardiography and superior in patients with poor acoustic windows
The use of dobutamine CMR is limited by the fact that the evaluation of contractile reserve has a reduced predictive accuracy if more severe ventricular dysfunction is present at rest
Contrast enhanced CMR appears to have a greater accuracy than dobutamine CMR, even in segments with severe dysfunction, does not require pharmacologic stress, involves less risk, is technically easier, and is less observer dependent for interpretation
If a cut‐off value of 25% transmural extent is used in the study by Kim et al,13 the positive and negative predictive values are 71% and 79%, respectively, for regions with any degree of dysfunction, and 88% and 89% for regions with akinesia or dyskinesia. Although these values are similar to those previously reported from traditional non‐invasive imaging techniques, the available data demonstrating a progressive relationship between the likelihood of wall motion improvement and the transmural extent of contrast enhancement clearly indicate that there is no single cut‐off value for contrast enhancement to predict functional improvement.
The knowledge of the transmural extent of infarction may have important diagnostic information apart from its use in the prediction of functional recovery. There is evidence suggesting that the presence of a viable epicardial rim might improve the outcome without increasing resting contractile function. Several authors showed that in patients with LV dysfunction the survival rates after coronary artery bypass surgery were similar among patients with ventricular dysfunction whether or not the function improved after the intervention. Thus, prognosis may be altered without changing LV function by improving the LV remodelling processes, preventing additional LV dilatation, promoting electrical stability, and reducing the risk of subsequent ischaemic events.
Recently, Wellnhofer et al14 compared ceCMR to dobutamine CMR for assessment of myocardial viability. Both techniques were performed in 29 patients with chronic CAD and resting LV dysfunction. Cine CMR imaging was performed three months after revascularisation to determine wall motion improvement. Using a cut‐off value of 25% transmural extent of infarction, the authors found a sensitivity and specificity of dobutamine CMR of approximately 75% and 93%, respectively, and a negative predictive value of 83%, indicating an advantage of dobutamine CMR compared to ceCMR. However, if a cut‐off value of 50% transmural extent was used, the negative predictive value for ceCMR was approximately 88%, which is similar to that found by Kim et al,13 who reported a negative predictive value of 92% using a cut‐off of 50%. Thus, for segments with more than 50% of scar extent, ceCMR predicts wall motion recovery after revascularisation with equal or higher confidence than dobutamine CMR.
Contractile reserve has a reduced predictive accuracy if more severe dysfunction is present at rest. In contrast, ceCMR appears to have a greater accuracy in segments with the most severe dysfunction.13 Further studies are needed to compare ceCMR and dobutamine CMR for different levels of LV dysfunction. However, ceCMR has the advantage that it does not require pharmacologic stress and thus involves less risk and subsequently less patient monitoring. Furthermore it is probably easier to undertake and less observer dependent for interpretation.
ASSESSMENT OF MYOCARDIAL ISCHAEMIA
Currently several CMR techniques are available for assessment of myocardial ischaemia. The most appealing approach to many cardiologists seems to be non‐invasive coronary MR angiography, which can be used to directly visualise coronary anatomy and morphology analogous to invasive x‐ray angiography or coronary CT‐angiography. However, this method is technically challenging and with current 1.5T scanners there are limitations in spatial and temporal resolution, which restrict the use of coronary MR angiography to the assessment of coronary anomalies and patency of aorto‐coronary bypass grafts.
DOBUTAMINE CMR
High dose dobutamine stress testing with CMR assessment of ventricular wall motion can also provide information concerning the presence and functional significance of coronary lesions.
Nagel et al15 were the first to directly compare high dose dobutamine CMR to an identical dobutamine echocardiography protocol using state of the art second harmonic echo imaging in the same group of 172 patients. The overall detection of CAD as defined by coronary angiography (diameter reduction >50%) was significantly better by CMR in terms of sensitivity (88.7% v 74.3%; p<0.05) and specificity (85.7% v 69.8%; p<0.05) as compared to stress echocardiography. The authors attributed the superior results of CMR to its better overall image quality, which was graded good or very good in 82% of CMR, but only in 51% of echocardiography studies (fig 1). This study using the same evaluation approach for CMR and echocardiography, based on identical segmentation of the left ventricle and qualitative criteria for wall motion analysis, convincingly demonstrated that high dose stress CMR is an accurate and effective tool for the detection of stress induced ischaemic wall motion abnormalities compared to the clinically well established dobutamine stress echocardiography.
Hundley et al16 performed a high dose dobutamine CMR protocol to test the utility of CMR for the detection of ischaemia in 153 patients who were not well suited for second harmonic stress echocardiography because of poor acoustic windows. This study reported a sensitivity and specificity of 83% for the detection of a coronary stenosis >50% luminal diameter.
Thus, high dose dobutamine CMR can detect ischaemia induced wall motion abnormalities and may be clinically useful in patients not suited for echocardiography because of poor acoustic windows.
PERFUSION CMR
Currently, stress perfusion CMR is less established for clinical application than high dose dobutamine CMR. However, there are convincing data that correlate perfusion CMR results with tissue perfusion in animal models17 and excellent correlations with radionuclide imaging and invasive x‐ray angiography in humans. Schwitter et al18 studied 48 patients with suspected CAD by perfusion CMR. Before coronary angiography, coronary flow reserve was determined in corresponding sectors by (13)N‐ammonia PET. Receiver operator characteristic revealed a sensitivity and specificity of 91% and 94% for CMR, respectively, for the detection of CAD as defined by PET (mean coronary flow reserve minus 2SD of controls), and a sensitivity and specificity of 87% and 85% for CMR, respectively, in comparison with quantitative coronary angiography (diameter stenosis ⩾50%).
However, there are few data so far to demonstrate the feasibility of stress perfusion CMR for everyday clinical use. Most published studies were either retrospective, required central venous lines, or acquired only one to two slices per heartbeat. Additionally, most stress perfusion CMR studies, including the study by Schwitter et al18 discussed above, required extensive interactive post‐processing, and quantitative image analysis, which reduces the applicability of this technique for everyday clinical routine.
Despite these limitations, the approach of perfusion CMR is still promising for several reasons:
Decreased perfusion is the first step in the cascade of myocardial ischaemia. Thus, techniques that assess perfusion have the potential to be more sensitive than techniques assessing later steps such as wall‐motion abnormalities.
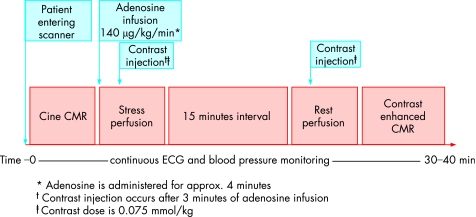
Perfusion CMR is quite quick and simple. We perform adenosine perfusion CMR as follows: After cine imaging, adenosine is administered at 140 μg/kg/min for 3 mins. At this time, the perfusion CMR scan is performed. Once the gadolinium bolus has transited the left ventricular myocardium, the adenosine is stopped, and imaging is completed. The total imaging time for perfusion CMR is maximum 45 s, and the total time of adenosine infusion is about 4 mins (fig 4).
The pulse sequences used for perfusion CMR are undergoing rapid evolution, and the technique will likely soon allow reliable visual interpretation for routine clinical use.
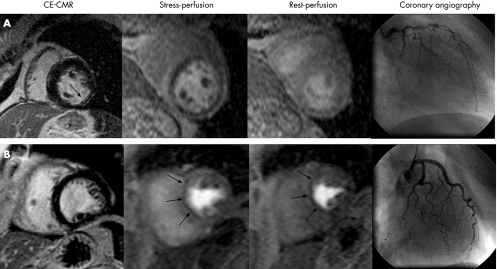
Perfusion CMR is quite demanding in terms of scanner hardware, since images are acquired in about 100 ms rather than built up over several cardiac cycles, which is the case for conventional cine and ceCMR. Thus, the signal‐to‐noise ratio is substantially lower for perfusion CMR, and even with the recent improvements, artefacts may reduce specificity. However, this limitation may have been overcome by the algorithmic approach used by Klem et al19 (fig 5). Based on the assumption that ceCMR is the most sensitive and specific CMR technique for the detection of infarction,13 perfusion defects that have similar intensity and extent during both stress and rest (“fixed defect”), but do not have contrast enhancement (no infarct), can now easily be identified as artefacts and should not be considered as being caused by CAD (figs 5 and 6). In this study, 40 of 92 patients had significant CAD (⩾70% stenosis). The combination of perfusion and ceCMR yielded a sensitivity, specificity, and accuracy of 89%, 87%, and 88%, respectively, for CAD diagnosis, compared with 84%, 58%, and 68% for perfusion CMR alone.19 This improvement was due to the exceptionally high specificity (98%) of ceCMR. Thus, combined perfusion and ceCMR examination using a visual interpretation algorithm can accurately diagnose CAD in a clinical setting and this combination is superior to perfusion CMR alone.
Figure 4 Schematic representation of a comprehensive stress cardiovascular magnetic resonance (CMR) examination consisting of cine imaging at rest, adenosine stress and rest perfusion imaging, and contrast enhanced imaging. Adapted from Willerson's textbook of cardiovascular medicine, 3rd ed, in print.
Figure 5 Interpretation algorithm for the diagnosis of coronary artery disease (CAD). (1) Positive contrast enhanced cardiovascular magnetic resonance (ceCMR) study: contrast enhanced myocardium consistent with a prior myocardial infarction (MI) is detected. This does not include isolated midwall or epicardial contrast enhancement which can occur in non‐ischemic disorders. (2) Standard negative stress study: no evidence of prior MI or inducible perfusion defects. (3) Standard positive stress study: no evidence of prior MI but perfusion defects are present with adenosine that are absent or reduced at rest. (4) Artefactual perfusion defect: matched stress and rest perfusion defects without evidence of prior MI on ceCMR. Adapted from Klem et al.19
Figure 6 Comprehensive CMR stress perfusion study. The upper panel depicts a positive ceCMR study on the left demonstrating subendocardial infarction in the inferolateral wall (single arrow). Consequently, this patient was ruled positive for CAD (see fig 5, step 1). Adenosine perfusion CMR revealed prominent reversible defects in all coronary artery territories (dark areas in stress perfusion), indicating myocardial ischaemia. Since the perfusion defects were significantly larger than the infarct, viable myocardium is at risk. Coronary angiography demonstrated multivessel disease in this patient, who then underwent successful surgical revascularisation. Panel B displays a patient with a matched stress–rest perfusion defect (arrows) but without evidence of prior MI on ceCMR (left bottom panel). Thus, the perfusion defects must be classified as artefactual and the patient as negative for CAD (see fig 5, step 4). Coronary angiography demonstrated normal coronary arteries.
Myocardial ischaemia: key points
For the clinical routine assessment of myocardial ischaemia, stress perfusion CMR and dobutamine CMR are promising alternatives to the more established nuclear and echocardiographic techniques
The assessment of perfusion by CMR has the potential to be more sensitive than dobutamine CMR, which assesses later steps in the cascade of ischaemia such as wall motion abnormalities
Perfusion CMR can be performed quickly and easily, and rapid evolution of CMR pulse sequences as well as establishment of combined imaging and interpretation algorithms will soon allow quick and easy visual interpretation
Only one study compared the relative merits of dobutamine CMR wall motion and adenosine CMR perfusion assessments for the diagnosis of CAD.20 Using a visual approach for CMR stress and rest perfusion image analysis, the authors concluded that adenosine stress CMR was inferior to dobutamine CMR because of its reduced specificity. As discussed above, we believe that the incorporation of ceCMR images can solve this problem. Thus, we think that both modes of stress testing are at least equal for the detection of CAD. Consequently, we prefer the combined CMR perfusion approach in our institution, since it easier to perform in clinical practice. Before making its way into the mainstream clinical routine, however, this stress perfusion CMR approach needs to be evaluated in larger prospective clinical trials.
SUMMARY
The diagnostic performance of dobutamine CMR for the assessment of myocardial viability is at least comparable to dobutamine echocardiography and superior in patients with poor acoustic windows. However, the use of dobutamine CMR for assessment of myocardial viability is limited by the fact that the evaluation of contractile reserve has a reduced predictive accuracy if more severe ventricular dysfunction is present at rest. In contrast, ceCMR appears to have a greater accuracy even in segments with most severe dysfunction. In addition, ceCMR does not require pharmacologic stress, involves less risk and subsequently less monitoring of the patient, and is technically easier as well as less observer dependent for interpretation. Thus, ceCMR should become the routine procedure for CMR assessment of myocardial viability.
For the clinical routine assessment of myocardial ischaemia, stress perfusion CMR and dobutamine CMR are promising alternatives to the more established nuclear and echocardiographic techniques. However, the assessment of perfusion by CMR has the potential to be more sensitive than dobutamine CMR, which assesses later steps in the cascade of ischaemia such as wall‐motion abnormalities. Furthermore, perfusion CMR can be performed quickly and easily, and rapid evolution of CMR pulse sequences as well as establishment of combined imaging and interpretation algorithms will soon allow quick and easy visual interpretation. Thus, we speculate that stress perfusion CMR will not only become a routine clinical procedure but also the dominant stress CMR modality in the future.
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
References
- 1.Baer F M, Voth E, Schneider C A.et al Comparison of low‐dose dobutamine‐ gradient‐echo magnetic resonance imaging and positron emission tomography with [18F]fluorodeoxyglucose in patients with chronic coronary artery disease. A functional and morphological approach to the detection of residual myocardial viability. Circulation 1995911006–1015. [DOI] [PubMed] [Google Scholar]
- 2.Gunning M G, Anagnostopoulos C, Knight C J.et al Comparison of 201Tl, 99mTc‐ tetrofosmin, and dobutamine magnetic resonance imaging for identifying hibernating myocardium. Circulation 1998981869–1874. [DOI] [PubMed] [Google Scholar]
- 3.Simonetti O P, Kim R J, Fieno D S.et al An improved MR imaging technique for the visualization of myocardial infarction. Radiology 2001218215–223.Initial study describing the use of the optimised segmented inversion recovery gradient recalled echo sequence for evaluation of myocardial infarction. [DOI] [PubMed] [Google Scholar]
- 4.Mahrholdt H, Wagner A, Holly T et a l. Reproducibility of infarct size measurements by contrast enhanced MRI. Circulation 20021062322–2327. [DOI] [PubMed] [Google Scholar]
- 5.Kim R J, Fieno D S, Parrish T B.et al Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 19991001992–2002.Landmark study comparing contrast enhancement to histopathology and concluding that contrast enhancement occurs in the setting of irreversible myocardial injury only. [DOI] [PubMed] [Google Scholar]
- 6.Ansari M, Araoz P A, Gerard S K.et al Comparison of late enhancement cardiovascular magnetic resonance and thallium SPECT in patients with coronary disease and left ventricular dysfunction. J Cardiovasc Magn Reson 20046549–556. [DOI] [PubMed] [Google Scholar]
- 7.Klein C, Nekolla S G, Bengel F M.et al Assessment of myocardial viability with contrast‐enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 2002105162–167. [DOI] [PubMed] [Google Scholar]
- 8.Kühl H P, Beek A M, van der Weerdt A P.et al Myocardial viability in chronic ischemic heart disease: comparison of contrast‐enhanced magnetic resonance imaging with (18)F‐fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2003411341–1348. [DOI] [PubMed] [Google Scholar]
- 9.Wagner A, Mahrholdt H, Holly T A.et al Contrast‐enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003361374–379.Landmark study concluding that ceCMR detects myocardial infarcts which are missed by SPECT imaging. [DOI] [PubMed] [Google Scholar]
- 10.Mahrholdt H, Wagner A, Parker M.et al Relationship of contractile function to transmural extent of infarction in patients with chronic coronary artery disease. J Am Coll Cardiol 200342505–512. [DOI] [PubMed] [Google Scholar]
- 11.Hillenbrand H B, Kim R J, Parker M A.et al Early assessment of myocardial salvage by contrast‐enhanced magnetic resonance imaging. Circulation 20001021678–1683. [DOI] [PubMed] [Google Scholar]
- 12.Choi K, Kim R J, Gubernikoff G.et al The transmural extent of acute myocardial infarction predicts long term improvement in contractile function. Circulation 20011041101–1107. [DOI] [PubMed] [Google Scholar]
- 13.Kim R J, Wu E, Rafael A.et al The use of contrast‐enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 20003431445–1453.The first study to demonstrate that ceCMR can accurately predict reversible myocardial dysfunction in patients with ischaemic heart disease before coronary revascularisation. [DOI] [PubMed] [Google Scholar]
- 14.Wellnhofer E, Olariu A, Klein C.et al Magnetic resonance low‐dose dobutamine test is superior to scar quantification for the prediction of functional recovery. Circulation 20041092172–2174. [DOI] [PubMed] [Google Scholar]
- 15.Nagel E, Lehmkuhl H B, Bocksch W.et al Diagnosis of ischemia‐induced wall motion abnormalities with the use of high‐dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation 199999763–770.Excellent study demonstrating that the diagnostic performance of dobutamine CMR is at least comparable to dobutamine echocardiography and superior in patients with poor acoustic windows. [DOI] [PubMed] [Google Scholar]
- 16.Hundley W G, Hamilton C A, Thomas M S.et al Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation 19991001676–1679. [DOI] [PubMed] [Google Scholar]
- 17.Klocke F J, Simonetti O P, Judd R M.et al Limits of detection of regional differences in vasodilated flow in viable myocardium by first‐pass magnetic resonance perfusion imaging. Circulation 20011042412–2416. [DOI] [PubMed] [Google Scholar]
- 18.Schwitter J, Nanz D, Kneifel S.et al Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation 20011032230–2235. [DOI] [PubMed] [Google Scholar]
- 19.Klem I, Heitner J F, Shah D J.et al Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol 2006471630–1638.The first study evaluating an algorithmic approach using perfusion CMR as well as ceCMR for the initial diagnosis of CAD. [DOI] [PubMed] [Google Scholar]
- 20.Paetsch I, Jahnke C, Wahl A.et al Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation 2004110835–842. [DOI] [PubMed] [Google Scholar]