Abstract
Objective
A previously developed pretest score was validated to stratify patients presenting for exercise testing with suspected coronary disease according to the presence of angiographic coronary disease. Our goal was to determine how well this pretest score risk stratified patients undergoing pharmacological and exercise stress tests concerning prognostic endpoints.
Design
Retrospective cohort analysis.
Setting
University hospital stress laboratory.
Patients
7452 unselected ambulatory patients with symptoms of suspected coronary disease undergoing stress testing between 1995 and 2004.
Main outcomes measures
All‐cause death, cardiac death and non‐fatal myocardial infarction.
Results
The rate of all‐cause death was 5.5% (CI 5.0 to 6.1) with 4.3 (SD 2.4) years of follow‐up (Exercise 2.8% (CI 2.3 to 3.2) v Pharmacological group 11.9% (CI 10.5 to 13.3); p<0.001). The rate of cardiac death/myocardial infarction was 2.6% (CI 2.2 to 3.0) (Exercise 1.4% (CI 1.1 to 1.8) v Pharmacological group 5.3% (CI 4.3 to 6.2); p<0.001). In both groups, stratification by pretest score was significant for all‐cause death and the combined endpoint. However, stratification was more effective in the pharmacological group using the combined endpoint rather than all‐cause death. Pharmacological stress patients in intermediate and high risk groups were at higher risk than their respective exercise test cohorts. Referral for pharmacological stress testing was found to be an independent predictor of time to death (2.7 (CI 2.0 to 3.6); p<0.001).
Conclusion
A pretest score previously validated to stratify according to angiographic outcomes, effectively risk stratified pharmacological and exercise stress patients according to the combined endpoint of cardiac death/myocardial infarction.
Keywords: coronary disease, pharmacological stress, prognosis
For patients with symptoms of suspected coronary artery disease, the process of risk stratification begins with the acquisition of a medical history. Several pretest scores designed to stratify patients into low and higher risk groups have been reported1,2,3,4 and endorsed by a number of published guidelines.2,5,6 To date, these reports have predominantly considered patients who underwent exercise testing; none have focused on patients who underwent pharmacological stress testing. Prior reports have demonstrated that in exercise stress patients with suspected coronary disease, a new pretest score accurately stratified into low and higher risk subgroups according to both angiographic disease presence and severity1 as well as prognostic outcomes.7,8 The following report is intended to assess the ability of this same pretest score to risk stratify patients with suspected coronary disease undergoing pharmacological as well as exercise stress testing.
Methods
Patient population
Between May 1995 and February 2004, we screened all out‐patients ⩾18 years of age referred by primary care physicians and cardiologists for their first stress test because of symptoms of suspected coronary disease. First stress tests included exercise electrocardiograms and exercise and pharmacological (adenosine, dipyridamole or dobutamine) imaging (nuclear perfusion or wall motion echocardiographic) studies. Patients referred for exercise imaging who were converted to pharmacological imaging due to inability to walk on the day of the treadmill test were analysed as pharmacological stress patients and those who were converted to pharmacological imaging due to inadequate exercise heart rate were analysed as exercise stress patients. Patients converted from pharmacological to exercise imaging were few and always due to clerical error in scheduling and were analysed as exercise stress patients. We included only symptomatic patients referred with the expressed purpose of evaluating for the presence of coronary disease. A significant number of the exercise test patients (n = 3975) were included in a prior report.7 Because of patient exclusions during the original derivation of the pretest score, we excluded patients with established coronary disease, those without presenting symptoms, and those with resting electrocardiograms considered uninterpretable (left ventricular hypertrophy, left bundle branch block, Wolff‐Parkinson‐White syndrome, or ⩾1 mm depression of the ST segment).5 These patients are being evaluated separately and will be the subject of future reports. Any subsequent reference to an abnormal electrocardiogram refers to abnormalities that would not affect the interpretability of the stress electrocardiogram, such as ST shifts <1 mm, isolated T inversions, and non‐specific non‐Q wave changes in the QRS complex including right bundle branch block, axis deviation, and abnormalities of R wave progression
Baseline clinical information
Data were collected from patients by a technician using a standard pre‐stress test questionnaire and confirmed by the physician supervising the stress test. We classified chest pain using the categories of Diamond.9 Risk factors included the following: current or prior cigarette smoking, history of hypertension (on antihypertensive therapy), history of insulin or non‐insulin requiring diabetes, history of high cholesterol or on cholesterol lowering therapy, a family history of premature (<60 years of age) coronary disease (infarction, coronary bypass or angioplasty, sudden death) in first degree relatives, and obesity defined as a body mass index (kg/m2) >27. Women were oestrogen status negative if they were postmenopausal and not receiving oestrogen replacement therapy. If they were premenopausal or receiving oestrogen replacement therapy, they were considered as oestrogen status positive. Women who had undergone hysterectomy without oopherectomy were considered oestrogen status positive if they were under the age of 50 and without symptoms of oestrogen deficiency. Otherwise, they were considered oestrogen status negative.10,11
Pretest score and determination of endpoints
Utilising the score presented in table 1, each patient was assigned to a low (0–8 points), intermediate (9–15 points) or high risk (>15 points) subgroup.1 These groupings are relevant to the recommendation of consensus guidelines5,6 to stratify in this manner prior to choosing an exercise test modality. Determination of endpoints occurred at least 1 year following the date of the stress test. Patients had vital status and date of death determined by a search of the Social Security Death Index. Cause of death was supplied as ICD‐9 or ICD‐10 codes by the West Virginia Department of Health and Human Resources. When cause of death could not be determined, patients were categorised as having non‐cardiac deaths. In addition, all patients' computerised medical records were reviewed concerning (i) the first non‐fatal acute myocardial infarction occurring after the stress test and (ii) the first percutaneous coronary intervention or coronary artery bypass surgery occurring after the stress test. Computer matches for the above endpoints were confirmed by visual inspection of the medical record. Myocardial infarction was documented by history, cardiac enzyme elevations and/or new Q waves on the electrocardiogram. Non‐fatal events occurring at other institutions were unlikely to be captured in this data review. Other significant non‐cardiac comorbidities as reflected by ICD‐9 codes available before and after the stress study were derived from the computerised hospital database. Approval for collection of all data was obtained from our institutional Human Subjects Committee.
Table 1 Pretest score calculation: how to calculate the pretest score and assigned risk groups.
| Variable | Choose response | Sum | ||
|---|---|---|---|---|
| Age | Men | Women | ||
| <40 | <50 | 3 | ||
| 40–54 | 50–64 | 6 | ||
| ⩾55 | ⩾65 | 9 | ||
| Oestrogen status | Positive = −3 | |||
| Women only | Negative = +3 | |||
| Angina history | Typical = 5 | |||
| Diamond method | Atypical = 3 | |||
| Non‐anginal = 1 | ||||
| Diabetes? | 2 | |||
| Hyperlipidaemia? | 1 | |||
| Hypertension? | 1 | |||
| Smoking? (any) | 1 | |||
| First degree family | 1 | |||
| history of CAD | ||||
| Obesity? BMI>27 | 1 | |||
| Total score: |
Statistical analysis
NCSS 2004 software (Number Cruncher Statistical System; www.ncss.com) was used for all statistical analyses. Comparison of frequencies was accomplished using χ2 testing. Comparison of means was accomplished using non‐paired t testing. Normality of data distribution was determined using the Wilk‐Shapiro test as well as observation of box and normal probability plots. When data were not distributed normally, the Mann Whitney U test was utilised.
Calibration was displayed in several ways depending on the intent with confidence intervals expressed as exact binomial confidence intervals. Calibration for all‐cause death was displayed by expressing event rates as a function of pretest probability group, the guideline sanctioned means of categorising risk. Patients who received revascularisation were removed from this calibration analysis, thereby removing the bias of the treatment effect on mortality. Calibration for cardiac death/non‐fatal myocardial infarction was displayed by both expressing event rates as a function of pretest probability group and visually plotting event rates as a function of the continuous pretest score. In this case, patients who received revascularisation were also removed from the calibration analysis unless a non‐fatal infarction occurred prior to the revascularisation. The Kaplan‐Meier method and Cox regression analysis were also utilised in survival analysis for assessing the time to an event. We censored at the first occurrence after stress testing of death with or without myocardial infarction, depending on the intent of the specific analysis. If revascularisation occurred prior to death or infarction, we censored at the time of revascularisation and the aforementioned event was ignored. p Values <0.05 were considered significant.
Results
Patient populations
During the period of interest, a total of 14 140 patients underwent their initial stress test. When those who were <18 years of age, inpatients or asymptomatic were removed, 10 172 patients remained. As an additional 2720 patients with known coronary disease, uninterpretable resting electrocardiograms or both were removed, 7452 patients with suspected coronary disease remained. A minority underwent the stress test to provide a preoperative evaluation (8.0% pharmacological and 0.9% exercise) or an evaluation of arrhythmias (0.8% pharmacological and 1.1% exercise), but the evaluation of reported symptoms of suspected coronary disease was an important consideration of the stress test. See table 2 for summary of clinical characteristics for the entire group as well as exercise and pharmacological subgroups.
Table 2 Clinical characteristics and endpoints.
| All | Exercise | Pharmacological | |
|---|---|---|---|
| Number | 7452 | 5156 | 2296 |
| Age | 51 (SD 14) | 48 (SD 12) | 58 (SD 14)* |
| Women (%) | 4098 (55) | 2638 (51) | 1460 (64)* |
| Oestrogen status negative (%) | 1566 (38) | 732 (28) | 834 (57)* |
| Symptoms | |||
| Typical (%) | 498 (7) | 340 (7) | 158 (7) |
| Atypical (%) | 3041 (41) | 2063 (40) | 978 (43) |
| Non‐anginal (%) | 3913 (52) | 2753 (53) | 1160 (50) |
| Diabetes (%) | 1154 (16) | 536 (10) | 618 (27)* |
| Smoking (%) | 3600 (48) | 2391 (46) | 1209 (53)* |
| Hyperlipidaemia (%) | 2401 (32) | 1556 (30) | 845 (37)* |
| Hypertension (%) | 3075 (41) | 1734 (34) | 1341 (58)* |
| Obesity (%) | 4801 (64) | 3205 (62) | 1596 (70)* |
| Family history (%) | 3591 (48) | 2537 (49) | 1054 (46)† |
| Pretest score | 10 (SD 5) | 9 (SD 4) | 12 (SD 4)* |
| New pretest score group | |||
| Low (%) | 2881 (39) | 2400 (47) | 481 (21) |
| Intermediate (%) | 3584 (48) | 2350 (46) | 1234 (54)* |
| High (%) | 987 (13) | 406 (7) | 581 (25) |
| All‐cause death | 431 (5.8, 1.3) | 154 (3.0, 0.7) | 277 (12, 2.8)* |
| Cardiac death | 151 (2.0, 0.5) | 43 (0.8, 0.2) | 108 (4.7, 1.1)* |
| Non‐fatal MI | 121 (1.6, 0.4) | 71 (1.4, 0.3) | 50 (2.2, 0.5)† |
| CD/MI | 264 (3.5, 0.8) | 109 (2.1, 0.5) | 155 (6.8, 1.6)* |
| Revascularisation | 440 (5.9, 1.4) | 258 (5.0, 1.2) | 182 (7.9, 1.8)* |
CD, cardiac death, MI, myocardial infarction; SD, standard deviation.
(n, n), (raw %, annualised %).
*p<0.001, †p<0.05 v Exercise group.
The pharmacological group was older, with a greater percentage of women and risk factors.
Prognostic endpoints
Follow‐up for all‐cause death was complete with an average of 4.3 years of follow‐up. Cause of death could not be determined in 20 of the 431 deaths (5%). Follow‐up for non‐fatal events through review of medical records was complete (all medical record numbers accounted for) with an average of 4.4 years of follow‐up. Table 2 displays the raw event rates for each of the endpoints. Without exception, the pharmacological group had a significantly greater frequency of bad endpoints. Calibration is reflected in table 3, which displays event rates (with confidence limits and annualised rates) for patients stratified by pretest probability groups. Event rates indicate significant stratification (p<0.001). However, the all‐cause death rates for the pharmacological group were significantly greater than their respective exercise counterparts. The annualised all‐cause death risk of 1.0% in the low risk pharmacological group is more comparable to the high risk exercise group at 0.8% than the low risk exercise group at 0.1%. The same was not true when the composite endpoint was used where the annualised event rate of 0.4% for the low risk pharmacological group is comparable to the low and intermediate risk exercise groups.
Table 3 Prognostic endpoints by pretest probability groups.
| Pretest risk group | n | Events | ACD | Events | CDMI | ||
|---|---|---|---|---|---|---|---|
| % (CI) | % Annualised | % (CI) | % Annualised | ||||
| Exercise | |||||||
| Low | 2372 | 19 | 0.8 (0.5 to 1.3) | 0.2 | 15 | 0.6 (0.4 to 1.0) | 0.1 |
| Intermediate | 2187 | 90 | 4.1 (3.3 to 5.1) | 1.0 | 44 | 2.0 (1.4 to 2.7) | 0.5 |
| High | 339 | 26 | 7.7 (5.2 to 11.2) | 1.8 | 12 | 3.5 (1.9 to 6.3) | 0.8 |
| Pharmacological | |||||||
| Low | 466 | 20 | 4.3 (2.7 to 6.7) | 1.0 | 8 | 1.7 (0.8 to 3.5) | 0.4 |
| Intermediate | 1129 | 150 | 13.3 (11.3 to 15.4) | 3.1 | 65 | 5.8 (4.5 to 7.3) | 1.4 |
| High | 519 | 82 | 15.8 (12.8 to 19.3) | 3.7 | 38 | 7.3 (5.3 to 10) | 1.7 |
ACD, all‐cause death; CDMI, cardiac death or myocardial infarction; CI, confidence interval.
Figure 1A–C expands on table 3 and displays a continuous pretest score calibration for predicting the composite endpoint. For all patients as well as the exercise and pharmacological subgroups, there was a stepwise increase in per cent risk and absolute events as the score increased. In fig 1, the low risk group (0–8 points) has consistently low event rates irrespective of the points accumulated. However, the intermediate risk group (9–15 points) demonstrated increasing risk as points increased. While this was true for both exercise and pharmacological stress patients, the pharmacological stress patients had higher event rates for each level of the score compared to the exercise stress patients. The number in the high risk group (>15 points) was relatively small, but the average risk in the high risk group was higher than the intermediate risk group. On average, for each risk subgroup, the pharmacological stress group carried at least twice the risk of the corresponding exercise group.
Figure 1 Pretest score calibration. Cardiac death/myocardial infarction is plotted as a function of the pretest score for all patients (A), exercise patients (B), and pharmacological stress patients (C). Vertical bars represent the event rate (dark horizontal line in bar) with the exact binomial confidence intervals. Each bar is accompanied by two numbers. The top number is the number of events and the bottom number is the total number of patients in the respective stratum. See text for further discussion.
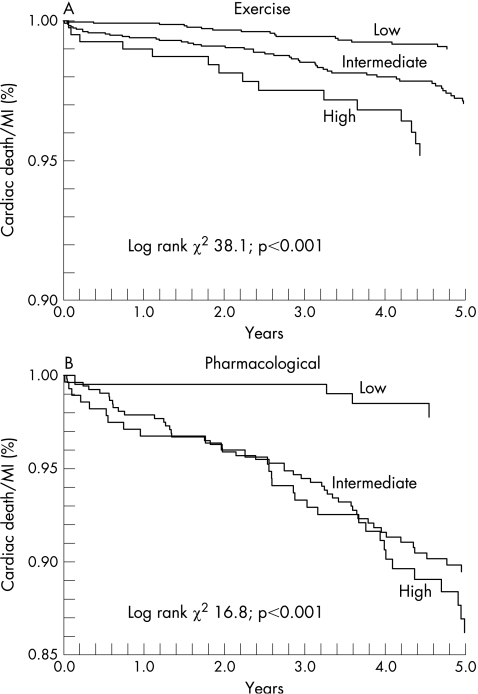
Figure 2 displays Kaplan‐Meier plots for exercise and pharmacological stress patients. The exercise patients show a clear stratification for the three risk groups. However, while the low risk pharmacological patients were clearly different from the other two groups, it was not possible to see a difference in risk between the intermediate and high risk pharmacological patients.
Figure 2 Kaplan‐Meier plots. Cardiac death/myocardial infarction is plotted as a function of the time to event for exercise (A) and pharmacological stress patients (B). See text for further discussion.
Survival analysis
In order to further explore the prognostic significance of pharmacological stress performance, we performed a multivariable Cox regression analysis. The variable “pharmacological stress” as a binary variable carried a highly significant (p<0.001) univariate risk ratio depending on the endpoint assessed (all‐cause death 5.7 (CI 4.4 to 7.4), cardiac death/myocardial infarction 3.6 (CI 2.6 to 4.9)). Using the continuous pretest score as a means to adjust for coronary risk reduced the risk ratios somewhat, but they were still highly significant (4.0 (CI 3.0 to 5.2) and 2.4 (CI 1.7 to 3.3), respectively). Adding β‐blocker presence and abnormal electrocardiogram to the model degraded the risk ratios further (3.7 (CI 2.8 to 4.8) and 2.1 (CI 1.5 to 3.0), respectively), but they were still highly significant. We added other comorbidities that had potential prognostic importance such as malignancy, chronic pulmonary disease, peripheral arterial disease (including cerebrovascular disease), renal disease, venous thromboembolic disease, anaemia, and other cardiac non‐coronary disease (including heart failure). After adjusting for all of these potential confounders, pharmacological stress was still a strong independent predictor of time to all‐cause death (2.7 (CI 2.0 to 3.6); p<0.001). Amongst the other variables, malignancy (2.5 (CI 1.9 to 3.3); p<0.001), lung disease (1.6 (CI 1.2 to 2.1); p = 0.002), anaemia (1.7 (CI 1.2 to 2.2); p = 0.001), renal disease (1.4 (CI 1.0 to 1.9); p = 0.03) and peripheral arterial disease (1.5 (CI 1.0 to 2.1); p = 0.04) were also significant independent predictors.
Discussion
The pretest score was originally developed and validated in an angiographic cohort to predict angiographic results.1 In a head to head comparison, it compared well to the familiar Diamond‐Forrester method for assigning angiographic estimates of prevalence.12 It differs from the Diamond‐Forrester method in that it allows for the consideration of coronary risk factors, especially diabetes, smoking, and hyperlipidaemia. The present study demonstrates that the new pretest score risk stratifies pharmacological stress as well as exercise stress patients presenting with symptoms of suspected coronary disease. Not surprisingly, given the greater frequency of coronary risk factors, pharmacological stress patients are at higher overall risk than comparable exercise stress patients. However, even after adjusting for coronary risk factors and other important comorbidities, pharmacological stress was an independent predictor of risk. Those pharmacological stress patients categorised as low risk are at low risk for cardiac events with risk similar to low pretest risk exercise test patients. Those categorised as greater than low risk have a gradient of risk that escalates as the pretest score increases. This gradient of risk appears to be steeper for those undergoing pharmacological stress. When compared to exercise test patients, all‐cause death was a less effective endpoint than the composite endpoint in patients undergoing pharmacological stress. The reason for this is that pharmacological stress patients are at greater risk of non‐cardiac death irrespective of the pretest coronary risk.
It has been known for some time that patients with poor exercise capacity have a poor prognosis.13 If we consider pharmacological stress to be a surrogate for poor functional capacity, our data further support this correlation. Our analysis indicates that even after adjusting for comorbidities such as cancer, emphysema, renal failure, heart failure, and stroke, pharmacological stress was still a strong predictor of death. This suggests that despite adjusting for these other illnesses, there are still missing covariates that are accounted for in the categorisation of pharmacological stress. Nevertheless, while pharmacological stress patients overall have significantly greater risk than exercise stress patients, the new pretest score presented here does define a subgroup within the pharmacological stress group that has low cardiac risk, that is <1%/year.
The clinical impact of a pretest score in the pharmacological stress population has yet to be defined. A report by Hachamovitch et al14 in a mixed group of patients with and without coronary disease illustrates how clinical risk stratification using a clinical score before pharmacological nuclear imaging might have a clinical impact. In patients undergoing adenosine myocardial perfusion studies, they demonstrated that adenosine stress studies further risk stratified for hard events those with a low pretest risk as well as those with intermediate–high pretest risk (pretest risk defined by a multivariable score named CADENZA). However, they noted that a minority of low risk patients were reclassified to higher levels of risk. This suggested to them that there may be a lack of cost‐effectiveness in testing low clinical risk patients.
Study limitations
While the findings of this study expand the use of the new pretest score to those undergoing pharmacological stress, these results cannot be applied to patients with known coronary disease, those with uninterpretable resting electrocardiograms (as we have defined this), those who are inpatients, and those without symptoms suggestive of coronary disease. There were 20 patients for whom cause of death could not be determined. In the foregoing analysis, these patients were categorised as having non‐cardiac deaths. However, when the analysis was conducted by classifying them as cardiac deaths, there was no significant change in the results. Because we used a hospital‐based medical record to assess for the non‐fatal endpoints of myocardial infarction and revascularisation, it is possible that some of those endpoints were missed in those patients who had those endpoints at other institutions.
Conclusion
A pretest score previously validated to stratify angiographic risk in exercise test populations appears to stratify prognostic risk in patients referred for pharmacological as well as exercise stress testing. This validation is limited to those without established coronary disease who present with symptoms in the outpatient setting. While guidelines suggest using risk categories (low, intermediate or high) for exercise test clinical decision‐making, this pretest score represents a continuous statement of risk. The implications of this are that patients within a given risk category have differing risk that varies according to both the actual precise pretest score and the method of stress. Further studies will be needed to incorporate “Pharmacological stress” into pretest scores and to develop risk stratification strategies specific for those who do not exercise.
Footnotes
Competing interests: None declared.
References
- 1.Morise A P, Haddad W J, Beckner D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med 1997102350–356. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons R J, Chatterjee K, Daley J.et al ACC/AHA/ACP‐ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Chronic Stable Angina). J Am Coll Cardiol 1999332092–2197. [DOI] [PubMed] [Google Scholar]
- 3.Pryor D B.et al Estimating the likelihood of severe coronary artery disease. Am J Med 199190553–562. [PubMed] [Google Scholar]
- 4.Kheng‐Thye H, Miller T D, Hodge D O.et al Use of a simple clinical score to predict prognosis of patients with normal or mildly abnormal resting electrocardiographic findings undergoing evaluation for coronary artery disease. Mayo Clin Proc 200277515–521. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons R J, Balady G J, Beasley J W.et al ACC/AHA guidelines for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol 199730260–315. [DOI] [PubMed] [Google Scholar]
- 6.Brindis R G, Douglas P S, Hendel R C.et al ACCF/ASNS appropriateness criteria for single‐photon emission computed tomography myocardial perfusion imaging (SPECT MPI). J Am Coll Cardiol 2005461587–1605. [DOI] [PubMed] [Google Scholar]
- 7.Morise A P, Jalisi F. Evaluation of pretest and exercise test scores to assess all‐cause mortality in unselected patients presenting for exercise testing with symptoms of suspected coronary artery disease. J Am Coll Cardiol 200342842–850. [DOI] [PubMed] [Google Scholar]
- 8.Morise A P, Olson M B, Bairey Merz C N.et al Validation of the accuracy of pretest and exercise test scores in women with a low prevalence of coronary disease: the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Am Heart J 20041471085–1092. [DOI] [PubMed] [Google Scholar]
- 9.Diamond G A. A clinically relevant classification of chest discomfort. J Am Coll Cardiol 19831574–575. [DOI] [PubMed] [Google Scholar]
- 10.Morise A P, Dalal J N, Duval R D. Value of a simple measure of estrogen status for improving the diagnosis of coronary artery disease in women. Am J Med 199394491–496. [DOI] [PubMed] [Google Scholar]
- 11.Morise A P, Haddad W J. Validation of estrogen status as an independent predictor of coronary artery disease presence and extent in women. J Cardiovasc Risk 19973507–511. [PubMed] [Google Scholar]
- 12.Morise A P. Comparison of the Diamond‐Forrester method and a new score to estimate the pretest probability of coronary disease prior to exercise testing. Am Heart J 1999138740–745. [DOI] [PubMed] [Google Scholar]
- 13.Morris C K, Ueshima K, Kawaguchi T.et al The prognostic value of exercise capacity: a review of the literature. Am Heart J 19911221423–1431. [DOI] [PubMed] [Google Scholar]
- 14.Hachamovitch R, Berman D S, Kiat H.et al Incremental prognostic value of adenosine stress myocardial perfusion single‐photon emission computed tomography and impact on subsequent management in patients with or suspected of having myocardial ischemia. Am J Cardiol 199780426–433. [DOI] [PubMed] [Google Scholar]