Abstract
Background
Cyclo‐oxygenase‐2 selective inhibitors have been associated with cardiovascular side effects, but previous studies have generally excluded people with previous myocardial infarction, thereby limiting our knowledge of their cardiotoxicity in this population.
Objectives
To determine whether a history of myocardial infarction modified the risk of acute myocardial infarction associated with the use of various non‐steroidal anti‐inflammatory drugs (NSAIDs).
Methods
A population‐based cohort of 122 079 elderly people with and without previous myocardial infarction newly treated with an NSAID between 1 January 1999 and 30 June 2002 were identified using the computerised health databases of Québec, Canada. A nested‐case–control approach was used for the analysis, with controls matched by cohort entry and age. Current users of NSAIDs, those whose last prescription overlapped with the index date, were compared with those who were not exposed to NSAIDs in the year preceding the event. Rate ratios of acute myocardial infarction were estimated using conditional logistic regression and adjusted for potential confounders.
Results
Users of rofecoxib, both with and without previous myocardial infarction, were at increased risk of myocardial infarction, with a trend for greater risk among those with a previous event (rate ratio (RR) 1.59, 95% confidence interval (CI) 1.15 to 2.18 v RR 1.23, 95% CI 1.05 to 1.45; p = 0.14 for interaction). By contrast, celecoxib was only associated with an increased risk in people with previous myocardial infarction (RR 1.40, 95% CI 1.06 to 1.84 v RR 1.03, 95% CI 0.88 to 1.20; p = 0.04 for interaction). The available power was insufficient to reliably assess risks among patients with previous myocardial infarction treated with other NSAIDs, dose–response relationships or interaction with aspirin.
Conclusions
Although only rofecoxib use was associated with an increased risk of myocardial infarction in those without a previous event, both rofecoxib and celecoxib were associated with an excess risk of acute myocardial infarction for current users with a history of myocardial infarction. A large randomised trial is required to more completely and reliably assess the cardiovascular safety of celecoxib and traditional NSAIDs in this population of high‐risk patients.
Randomised trials involving cyclo‐oxygenase‐2 (Cox‐2) selective inhibitors rofecoxib,1,2 celecoxib,3 valdecoxib4,5 and even the traditional non‐selective non‐steroidal anti‐inflammatory drug (NSAID) naproxen6 have all reported increased cardiovascular risks. However, several observational studies7,8,9,10,11 have found an increased risk with rofecoxib but not with celecoxib (valdecoxib being incompletely studied owing to its more recent marketing), and these results are in agreement with other randomised trials on celecoxib that failed to show cardiovascular risk.12,13 This disparate body of evidence and public concern on drug safety resulted in a joint public meeting of the Food and Drug Administration Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee,14 which while acknowledging the associated cardiovascular risks of Cox‐2 inhibitors, emphasised the need for further research.
An important question concerns the risk of Cox‐2 inhibitors in patients with definite pre‐existing cardiovascular disease, as previous studies have generally excluded such patients. Although established cardiovascular disease is a highly prevalent condition, especially in the same elderly people likely to be exposed to NSAIDs, including Cox‐2 inhibitors, a randomised clinical trial has not been conducted in this population. Consequently, using population databases, we specifically sought to determine whether a history of myocardial infarction modified the risk of acute myocardial infarction associated with the use of NSAIDs.
Methods
Subjects and data source
The details of our study design have been published previously.9 Briefly, a cohort of elderly subjects newly treated with an NSAID between 1999 and 2002 was identified using the computerised databases of the universal health insurance programmes of the province of Québec, Canada. The databases provided individual‐level information on sociodemographics and dates of coverage, as well as information on all drugs dispensed on an outpatient basis, doctor visits and hospitalisations. The vital statistics database was used to obtain information on dates and causes of death. The study was approved by both provincial and local ethical boards (Commission d'accès à l'information du Québec and the Royal Victoria Hospital, McGill University).
Study design
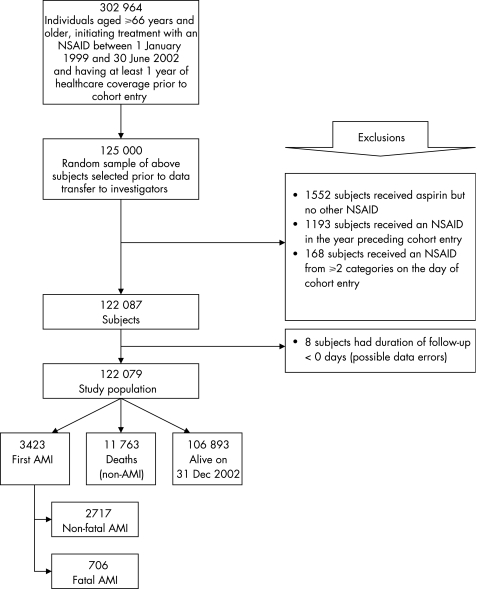
We conducted a population‐based, retrospective cohort study that was analysed using a time‐matched, nested case–control approach. The study population consisted of a random sample (n = 125 000) of all residents aged ⩾66 years who were dispensed an NSAID between 1 January 1999 and 30 June 2002. The date of the first such prescription was taken as date of cohort entry. Subjects were excluded if they had not been enrolled in the provincial health plan for at least 1 year preceding cohort entry, had been dispensed a study drug within this 1‐year baseline period (n = 1193), had received aspirin but no other NSAID (n = 1552) or had received prescriptions from two or more NSAID categories on the day of cohort entry (n = 168). The remaining subjects were followed up until the date of first hospitalisation for an acute myocardial infarction, death, the end of health coverage (due to emigration from the province) or 31 December 2002 (end of study), whichever came first.
Outcome
The case‐defining event was the first hospitalisation with a diagnosis of acute myocardial infarction (International Classification of Diseases, ninth edition, code 410), non‐fatal or fatal, occurring any time after cohort entry, and the date of admission was taken as the index date. The myocardial infarction was considered fatal if the person died within 30 days of admission. The coding accuracy of this diagnostic code in these databases has previously been validated.15 In order for non‐fatal myocardial infarction to be considered a valid outcome of the study, the length of stay at a hospital had to be at least 3 days, unless the person had been transferred to or from another institution or had undergone percutaneous coronary angioplasty.
Anti‐inflammatory drug exposure
All NSAIDs commercially available during the study period were classified according to their differential inhibition of Cox‐1 and Cox‐2 as follows:
non‐aspirin NSAIDs,
naproxen,
celecoxib,
rofecoxib and
meloxicam.
These agents, as well as over‐the‐counter aspirin and ibuprofen, were covered by the prescription drug plan and available with no restrictions of prescription. The accuracy and completeness of the prescription drugs database has been shown previously.16 The exposure time‐window of primary interest was current use, defined by drug exposure on the index date and operationalised by requiring that the duration of the last prescription dispensed overlapped with the index date. Past users had filled at least one prescription for an NSAID in the year preceding the index date, but were not currently exposed. Those without an NSAID prescription in this 1‐year period were considered to be non‐users.
We defined two dosage categories a priori for the Cox‐2 inhibitors: low dose (⩽200 and ⩽25 mg/day for celecoxib and rofecoxib, respectively) and high dose (>200 and >25 mg/day for celecoxib and rofecoxib, respectively). To study the effects of aspirin on the association between NSAIDs and myocardial infarction, we classified the subjects as current users of aspirin if the duration of the last prescription dispensed overlapped with the index date or ended within 30 days of this date. The 30‐day grace period was used to account for aspirin's irreversible inhibition of platelet aggregation, as well as possible alternate‐day prescriptions.
Data analysis
The choice and use of drugs often change over time; consequently, exposure to drugs needs to be analysed as a time‐dependent variable. To study the exposure to NSAIDs in relation to the date of hospitalisation for an acute myocardial infarction (ie, the aetiologically relevant exposure time window), while simultaneously controlling for the potentially confounding effects of calendar time, a time‐matched, nested case–control analysis was used.17,18 For each case‐defining event, up to 20 controls, matched by month and year of cohort entry and age (±1 year), were randomly selected from the case's risk set and assigned the same index date. In this way, controls (ie, non‐cases) were selected from the same cohort of NSAID users as cases, thus maximising the comparability of these two groups. The primary analysis was based on current exposure but past use was also assessed. To disentangle the independent effect of individual Cox‐2 inhibitors from those of naproxen and other NSAIDs, as well as the independent contribution of past use, we compared the risk of acute myocardial infarction associated with the current use of these agents with that of non‐users (ie, unexposed) in the year preceding the index date (reference category). We estimated the rate ratios (RRs) for these associations using conditional logistic regression to account for individual‐level matching19,20 and adjusted for the potentially confounding effects of conventional risk factors, including age, sex, hypertension, pre‐existing coronary artery disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure, diabetes, use of antilipidaemic agents, anticoagulants and aspirin. As our previous study showed no gain in precision with the use of a parsimonious model, all RRs were also adjusted for the presence of respiratory illness, gastrointestinal ulcer disease, thyroid disorders, depression or psychiatric illness, the use of oral corticosteroids, three measures of healthcare utilisation (the number of hospitalisations, medical outpatient visits and visits to a cardiologist), as well as three measures of comorbidity: the chronic disease score,21 the number of distinct drugs dispensed22 and the Charlson index.23 Information on risk factors, comorbid conditions, drug and healthcare utilisation was obtained from the various databases for all cases and controls. To evaluate the potential risk‐modifying effects of a previous myocardial infarction, an interaction term for a previous event was included with each exposure category in the primary analysis. The potential risk‐modifying effect of current use of aspirin was also studied using an interaction term in subsequent models.
We also undertook some sensitivity analyses. To test the robustness of the reference category chosen and of the use of a grace period for defining current users of aspirin, we repeated the analyses using people who were current non‐users as the reference group and changing the aspirin grace period to 15 days.
Results
Treatment with an NSAID was initiated in 302 964 elderly people during the study period (fig 1), but the provincial ethics review board authorised transfer to the investigators of only a random sample of 125 000 people. After applying the exclusion criteria, the study population consisted of 122 079 people, with a mean (standard deviation (SD)) age of 75.3 (5.6) years at cohort entry, who were followed up for an average of 2.3 (0.99) years. During this period, 3423 people were hospitalised for a myocardial infarction, of which 706 (20.6%) were fatal.
Figure 1 Study population. AMI, acute myocardial infarction; NSAID, non‐steroidal anti‐inflammatory drug.
In the year preceding the index date, 71.4% of cohort members received at least one NSAID prescription, representing 51.9% past users and 19.5% current users. The remaining 28.6% were non‐users during this time period (reference category). Among those currently exposed, the mean (SD) number of prescriptions dispensed in the year preceding the index date was 4.0 (5.4) for traditional NSAIDs, 3.0 (4.2) for naproxen, 6.1 (6.2) for celecoxib, 5.2 (6.0) for rofecoxib and 4.2 (3.7) for meloxicam, with an average duration of 28 days/prescription. For users of celecoxib, 74.1% received doses of ⩽200 mg/day, 25.8% received 400 mg/day and only 0.1% received doses of 800 mg/day. Users of rofecoxib also received predominantly low doses, as >90% of these people were prescribed ⩽25 mg/day; >95% of those dispensed aspirin received a daily dose of ⩽325 mg.
During follow‐up, 3423 cases and 68 456 controls were identified, thus a few cases did not have 20 perfectly matching controls. Table 1 outlines the characteristics of these cases and controls. As expected, cases were more likely to be men, having other manifestations of atherosclerosis including previous myocardial infarction, having more traditional cardiovascular risk factors and seemed generally more sick than controls. These differences were controlled for in the analysis to ensure the comparability of cases and controls.
Table 1 Characteristics of cases and controls.
| Cases (n = 3423) | Controls§ (n = 68 456) | |
|---|---|---|
| Mean (SD) age*, years | 78.2 (5.4) | 78.2 (5.4) |
| Sex (%) | ||
| Female | 52.1 | 67.1 |
| Male | 47.9 | 32.9 |
| Comorbid conditions (%)† | ||
| Hypertension | 57.0 | 50.1 |
| Coronary artery disease | 38.4 | 19.9 |
| Cerebrovascular disease | 2.0 | 0.9 |
| Peripheral vascular disease | 4.9 | 1.6 |
| Previous MI | 16.9 | 6.2 |
| Congestive heart failure | 19.3 | 8.4 |
| Diabetes | 25.4 | 11.8 |
| Respiratory illness | 26.4 | 19.1 |
| Gastrointestinal ulcer disease | 28.8 | 24.6 |
| Thyroid disorders | 17.0 | 16.9 |
| Depression/psychiatric illness | 15.3 | 14.6 |
| Cancer | 3.1 | 2.7 |
| Use of concomitant treatment (%)† | ||
| Use of antilipidaemic agents | 26.4 | 20.7 |
| Use of anticoagulants | 7.2 | 4.8 |
| Use of low‐dose ASA | 41.2 | 24.4 |
| Use of oral corticosteroids | 9.5 | 6.6 |
| Healthcare utilisation‡ | ||
| Hospitalisations (%) | ||
| None | 59.2 | 75.1 |
| ⩾1 | 40.8 | 24.9 |
| Outpatient medical visits | ||
| All visits to the doctor (%) | ||
| ⩽12 | 61.8 | 71.8 |
| >12 | 38.2 | 28.2 |
| Visits to the cardiologist (%) | ||
| None | 72.1 | 83.3 |
| ⩾1 | 27.9 | 16.7 |
| Comorbidity indices‡ | ||
| Mean (SD) of different drugs | 11.5 (6.4) | 8.6 (5.3) |
| Mean (SD) chronic disease score | 7.7 (4.3) | 5.6 (4.0) |
| Mean (SD) Charlson index | 0.89 (1.8) | 0.38 (1.2) |
ASA, acetylsalicylic acid; MI, myocardial infarction.
*At index date.
†In the year preceding initiation of an anti‐inflammatory agent (ie, cohort entry).
‡In the year preceding the index date.
§Four controls could not be found.
After adjusting for multiple risk factors, current users of rofecoxib, compared with non‐users in the year preceding the index date, were at an increased risk of having an acute myocardial infarction regardless of the presence of a previous event. A trend for a twofold increase in risk was observed among people with previous myocardial infarction (RR, 1.59, 95% CI 1.15 to 2.18) than among those without (RR 1.23, 95% CI 1.05 to 1.45; table 2), although this did not reach significance (p = 0.14). By contrast, celecoxib did not increase the risk of myocardial infarction among those without a previous myocardial infarction (RR 1.03, 95% CI 0.88 to 1.20), but did increase the risk for those with a previous myocardial infarction (RR 1.40, 95% CI 1.06 to 1.84), and the difference between these two groups was significant (p = 0.04). Subjects currently exposed to traditional NSAIDs or naproxen did not seem to be at an increased risk (RR 1.00, 95% CI 0.75 to 1.34 and RR 1.24, 95% CI 0.83 to 1.84, respectively) regardless of their cardiac history. There were too few users of meloxicam to assess the risk modifying effect of a previous myocardial infarction. The results of the sensitivity analysis using subjects who were current non‐users as the reference group were nearly identical to those of the primary analysis.
Table 2 Unadjusted and adjusted rate ratios of acute myocardial infarction for current use of various non‐steroidal anti‐inflammatory drugs according to the presence of a previous myocardial infarction.
| Cases (n = 3423) | Controls (n = 68 456) | Unadjusted RR | Adjusted* RR (95% CI) | p Value† | |
|---|---|---|---|---|---|
| No use‡ (%) | 932 (27.2) | 19 647 (28.7) | 1.00 | 1.00 (reference) | Reference |
| Current use (%) | |||||
| NSAIDs | 59 (1.7) | 1191 (1.7) | 1.10 | 1.00 (0.75 to 1.34) | |
| No previous MI | 51 | 1123 | 1.01 (0.74 to 1.38) | 0.87 | |
| Previous MI | 8 | 68 | 0.95 (0.44 to 2.04) | ||
| Naproxen | 31 (0.9) | 451 (0.7) | 1.54 | 1.24 (0.83 to 1.84) | |
| No previous MI | 23 | 423 | 1.18 (0.75 to 1.84) | 0.55 | |
| Previous MI | 8 | 28 | 1.56 (0.68 to 3.58) | ||
| Rofecoxib | 297 (8.7) | 4603 (6.7) | 1.43 | 1.28 (1.10 to 1.49) | |
| No previous MI | 239 | 4295 | 1.23 (1.05 to 1.45) | 0.14 | |
| Previous MI | 58 | 308 | 1.59 (1.15 to 2.18) | ||
| Celecoxib | 369 (10.8) | 6793 (9.9) | 1.19 | 1.08 (0.94 to 1.25) | |
| No previous MI | 287 | 6321 | 1.03 (0.88 to 1.20) | 0.04 | |
| Previous MI | 82 | 472 | 1.40 (1.06 to 1.84) | ||
| Meloxicam | 7 (0.2) | 180 (0.3) | 0.84 | 0.78 (0.36 to 1.68) | |
| No previous MI | 7 | 172 | 0.88 (0.41 to 1.91) | — | |
| Previous MI | 0 | 8 | — | ||
| Past use (%)§ | 1728 (50.5) | 35591 (52.0) | 1.05 | 0.95 (0.86 to 1.05) |
MI, myocardial infarction; NSAIDs, non‐steroidal anti‐inflammatory drugs.
*Adjusted for age at index (continuous variable); sex, hypertension, coronary artery disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure, diabetes, respiratory illness, gastrointestinal ulcer disease, thyroid disorders, depression/psychiatric illness and cancer, in the year preceding cohort entry; use of concomitant treatment including antilipidaemic agents, anticoagulants and aspirin in the year preceding cohort entry; healthcare utilisation including hospitalisations, outpatient visits to any doctor and outpatient cardiologist visits, in the year preceding the index date; and number of different drugs taken, chronic disease score and Charlson index in the year preceding the index date. RRs are for current users compared with no use in the year preceding the index date.
†For two‐sided test of interaction comparing previous MI and no previous MI at a significance level of α = 0.05.
‡No use in the year preceding the index date.
§Past users are those who were currently unexposed but had received at least one prescription for an NSAID in the year preceding the index date.
Although the point estimate of the risk associated with current use of rofecoxib was increased at higher doses for both subjects with (RR 2.99, 95% CI 1.25 to 7.14) and without a history of myocardial infarction (RR 1.66, 95% CI 1.04 to 2.63), this did not reach statistical significance compared with use of low doses (table 3). Among users of celecoxib, the risk for high dose (RR 1.60, 95% CI 1.00 to 2.54) was again not significantly different from low dose (RR 1.32, 95% CI 0.96 to 1.82). Although the coadministration of aspirin decreased the point estimates of the risk for all scenarios, these did not reach statistical significance (table 4). The pattern of effect modification of aspirin was unchanged in the sensitivity analyses using a 15‐day grace period for the definition of current users of aspirin.
Table 3 Adjusted rate ratios of acute myocardial infarction for current use of celecoxib and rofecoxib according to the presence of a previous myocardial infarction and the dose prescribed.
| No previous MI | Previous MI | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Adjusted RR* (95% CI) | p Values | Cases | Controls | Adjusted RR* (95% CI) | p Values | |
| No use† | 793 | 18502 | 1.00 (reference) | 139 | 1145 | 1.00 (reference) | ||
| Celecoxib | 287 | 6321 | 1.03 (0.88 to 1.20) | 82 | 472 | 1.40 (1.06 to 1.84) | ||
| Low dose‡ | 208 | 4704 | 1.01 (0.86 to 1.20) | 0.75 | 57 | 349 | 1.32 (0.96 to 1.82) | 0.50 |
| High dose§ | 79 | 1617 | 1.06 (0.83 to 1.36) | 25 | 123 | 1.60 (1.00 to 2.54) | ||
| Rofecoxib | 239 | 4295 | 1.23 (1.05 to 1.45) | 58 | 308 | 1.59 (1.15 to 2.18) | ||
| Low dose‡ | 218 | 4010 | 1.20 (1.02 to 1.42) | 0.20 | 50 | 287 | 1.48 (1.06 to 2.07) | 0.14 |
| High dose§ | 21 | 285 | 1.66 (1.04 to 2.63) | 8 | 21 | 2.99 (1.25 to 7.14) | ||
MI, myocardial infarction.
*Adjusted for age at index (continuous variable); sex, hypertension, coronary artery disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure, diabetes, respiratory illness, gastrointestinal ulcer disease, thyroid disorders, depression/psychiatric illness and cancer, in the year preceding cohort entry; use of concomitant therapy including antilipidaemic agents, anticoagulants, aspirin and past use of non‐steroidal anti‐inflammatory drugs, in the year preceding cohort entry; healthcare utilisation including hospitalisations, outpatient visits to any doctor and outpatient cardiologist visits, in the year preceding the index date; number of different drugs taken, chronic disease score and Charlson index in the year preceding the index date. Rate ratios are for current users compared with no use in the year preceding the index date.
†No use in the year preceding the index date.
‡⩽200 and ⩽25 mg/day for celecoxib and rofecoxib, respectively.
§>200 and >25 mg/day for celecoxib and rofecoxib, respectively.
Table 4 Adjusted rate ratios of acute myocardial infarction for current use of celecoxib and rofecoxib according to the presence of a previous myocardial infarction and the concomitant use of aspirin.
| No previous MI | p Value† | Previous MI | p Value† | |||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Adjusted RR* (95% CI) | Cases | Controls | Adjusted RR* (95% CI) | |||
| No use‡ | 793 | 18502 | 1.00 (reference) | Reference | 139 | 1145 | 1.00 (reference) | Reference |
| Celecoxib | ||||||||
| No aspirin | 204 | 4953 | 1.11 (0.94 to 1.32) | 0.07 | 37 | 236 | 1.59 (1.17 to 2.18) | 0.07 |
| Aspirin use | 83 | 1368 | 0.88 (0.70 to 1.11) | 45 | 236 | 1.27 (0.94 to 1.71) | ||
| Rofecoxib | ||||||||
| No aspirin | 158 | 3295 | 1.30 (1.08 to 1.57) | 0.25 | 27 | 148 | 1.75 (1.23 to 2.50) | 0.25 |
| Aspirin use | 81 | 1000 | 1.12 (0.88 to 1.42) | 31 | 160 | 1.50 (1.07 to 2.09) | ||
MI, myocardial infarction.
*Adjusted for age at index (continuous variable); sex, hypertension, coronary artery disease, cerebrovascular disease, peripheral vascular disease, previous MI, congestive heart failure, diabetes, respiratory illness, gastrointestinal ulcer disease, thyroid disorders, depression/psychiatric illness and cancer, in the year preceding cohort entry; use of concomitant treatment including antilipidaemic agents, anticoagulants, aspirin and use of other non‐steroidal anti‐inflammatory drugs in the year preceding cohort entry; healthcare utilisation including hospitalisations, outpatient visits to any doctor and outpatient cardiologist visits, in the year preceding the index date; number of different drugs taken, chronic disease score and Charlson index in the year preceding the index date. Rate ratios are for current users compared with no use in the year preceding the index date.
†For two‐sided test of interaction comparing use and no use of aspirin at a significance level of α = 0.05, stratified according to the presence of a previous MI.
‡No use in the year preceding the index date.
Discussion
Our study is the first to examine in detail the effect modification of a previous myocardial infarction on the cardiac risk associated with the use of Cox‐2 inhibitors. Our results show that current users of celecoxib with a history of myocardial infarction, in contrast with those without such a history, have a significantly increased risk of a subsequent myocardial infarction. Rofecoxib was also associated with an increased cardiovascular risk among those with a history of myocardial infarction, although this did not reach statistical significance compared with those without this history. Notably, only rofecoxib was associated with an increased risk among people with no history of myocardial infarction. Our study was unable to reliably confirm a dose–response relationship with these increased risks, perhaps owing to a lack of sufficient power.
Several distinctions are apparent in our observed risk profiles between celecoxib and rofecoxib. Firstly, the point estimates for celecoxib risk were systematically less than for rofecoxib, regardless of dose and patient profile, suggesting a higher risk with rofecoxib. Secondly, celecoxib increased the coronary risk only in users with a previous myocardial infarction. Our previous study9 of a population with no history of myocardial infarction did not detect a significant risk for celecoxib (RR 0.99, 95% CI 0.85 to 1.16), highlighting the importance of examining the cardiovascular risk profile of the population studied when interpreting Cox‐2 inhibitor study results. Although our study population had a higher cardiovascular risk profile than the Adenoma Prevention with Celecoxib population,3 it was generally exposed to much lower doses of celecoxib. Therefore, it seems that differing population risk gradients and dosing regimens may explain the divergent results of previously published trials, especially for celecoxib.3,12
In the past year, there has been intense research activity assessing the cardiovascular safety of Cox‐2 inhibitors, but mostly dealing with subjects who were free of pre‐existing clinically relevant cardiovascular disease. However, in routine practice, many people requiring NSAIDs are likely to have pre‐existing cardiac disease. Two studies on valdecoxib after coronary artery bypass surgery4,5 showed increased risk, further emphasising the need for further research into the cardiovascular safety profile of other Cox‐2 inhibitors among patients with established coronary artery disease. This is the first observational study to specifically assess the coronary risk of celecoxib and rofecoxib in such a high‐risk population, as defined by the occurrence of a previous myocardial infarction. For example, although the Adenomatous Polyp Prevention on Vioxx Trial confirmed increased cardiotoxicity with rofecoxib as compared with placebo (RR 1.92, 95% CI 1.19 to 3.11; p = 0.008), there was insufficient power to examine subgroups at high risk as only 9% of the 2586 randomised patients had symptomatic atherosclerotic cardiovascular disease (RR 9.59, 95% CI 1.36 to 416).2 Our study provides evidence that the presence of pre‐existing coronary disease seems to increase the cardiovascular risk profile associated with Cox‐2 inhibitors.
Our observed patterns of risk are consistent with the prevailing hypothesis that cardiovascular toxicity of Cox‐2 selective inhibitors is due to an unbalancing of the prostacyclin/thromboxane equilibrium and the known pharmacodynamic differences between these agents. The Cox‐2 inhibiting potency of celecoxib is nearly 10 times less than that of rofecoxib,24 and may explain why the magnitude of celecoxib risk is consistently less than that observed with rofecoxib, and present only in patients at high risk. It would seem that for potentially highly thrombogenic agents such as rofecoxib, the risk of myocardial infarction is increased regardless of cardiac history, but that for less thrombogenic agents such as celecoxib, the cardiovascular risk profile of an individual becomes an important modifier of cardiotoxicity.
The limitations of our study should be acknowledged. Firstly, although we did not observe any increased risk among people with a previous myocardial infarction with the use of naproxen, traditional NSAIDs or meloxicam, our power to detect meaningful differences was limited by the unexpectedly low usage of these agents. Secondly, only patients with myocardial infarctions admitted to the hospital could be considered in our analysis as cases. Missing events owing to silent myocardial infarctions and sudden death could have resulted in incomplete case ascertainment. Also, the intermittent use that occasionally accompanies NSAID treatment may have led to misclassification of exposure. However, there is no reason to believe that ascertainment or exposure errors would have occurred differentially across drug groups. Thirdly, we did not have information on smoking status, obesity, physical activity, family history and socioeconomic status. If the distribution of these risk factors varied considerably across exposure groups, our results could be biased owing to confounding by indication. Another potential limitation was our inability to account for over‐the‐counter use of aspirin and ibuprofen. However, as we have discussed in our previous publication,9 in the Québec context, these biases are likely to be negligible and any resulting bias would again be directed towards the null, leading to an underestimation of the true risks. Moreover, given that the only source of losses to follow‐up in our study was emigration of beneficiaries from the province and the older age of our study population, losses to follow‐up would have been extremely low. Our results should not be extrapolated to younger populations that have not been studied.
It is also important to consider absolute risks to assess the effect of our findings on public health. In our previous study of people without previous myocardial infarction,9 the excess myocardial infarction rate attributed to low‐dose and high‐dose rofecoxib was estimated at 2.2 and 7.7 myocardial infarctions per 1000 exposed elderly adults, respectively. In the present cohort of elderly adults with a history of myocardial infarction, the baseline annual myocardial infarction rate is threefold higher (35/1000), and assuming causality for our observed associations, rofecoxib and celecoxib may be responsible for an excess 21 and 14 myocardial infarctions per 1000 patients exposed.
Conclusions
Our results provide evidence that the risk of myocardial infarction associated with rofecoxib is approximately doubled by a history of myocardial infarction. Celecoxib was also associated with an increase in the risk of myocardial infarction, but only in those with a history of such an event. Therefore our results, although compatible with the prevailing opinion that the cardiotoxic effects of Cox‐2 inhibitors are a class effect, do suggest the presence of several nuances to this claim, including the importance of the molecule, dosage and overall cardiovascular risk profiles of patients. However, the discordance among previous randomised trials on celecoxib and our limited power to assess the cardiovascular risk of traditional NSAIDs, the risk‐modifying effect of aspirin, combined with the possibility of residual confounding in observational studies, supports the concept of a large randomised trial of celecoxib and traditional NSAIDs in a similar population at high risk.25
Abbreviations
Cox‐2 - cyclo‐oxygenase‐2
NSAID - non‐steroidal anti‐inflammatory drug
Footnotes
Funding: This study was funded by a grant from the Canadian Institutes of Health Research (CIHR grant MOP62871), who had no role in the design, conduct, interpretation or reporting of the study or the decision to submit the manuscript for publication.
Competing interests: None.
References
- 1.Bombardier C, Laine L, Reicin A.et al Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 20003431520–1528. [DOI] [PubMed] [Google Scholar]
- 2.Bresalier R S, Sandler R S, Quan H.et al Cardiovascular events associated with rofecoxib in a Colorectal Adenoma Chemoprevention Trial. N Engl J Med 20053521092–1102. [DOI] [PubMed] [Google Scholar]
- 3.Solomon S D, McMurray J J V, Pfeffer M A.et al Cardiovascular risk associated with celecoxib in a Clinical Trial for Colorectal Adenoma Prevention. N Engl J Med 20053521071–1080. [DOI] [PubMed] [Google Scholar]
- 4.Ott E, Nussmeier N A, Duke P C.et al Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 20031251481–1492. [DOI] [PubMed] [Google Scholar]
- 5.Nussmeier N A, Whelton A A, Brown M T.et al Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 20053521081–1091. [DOI] [PubMed] [Google Scholar]
- 6.Anon Washington Post: Aleve ingredient joins painkillers linked to risks, 2005. http://www.washingtonpost.com/wp‐dyn/articles/A14596‐2004Dec20.html (accessed 10 Oct 2006)
- 7.Solomon D H, Schneeweiss S, Glynn R J.et al Relationship between selective cyclooxygenase‐2 inhibitors and acute myocardial infarction in older adults. Circulation 20041092068–2073. [DOI] [PubMed] [Google Scholar]
- 8.Ray W A, Stein C M, Daugherty J R.et al COX‐2 selective non‐steroidal anti‐inflammatory drugs and risk of serious coronary heart disease. Lancet 20023601071–1073. [DOI] [PubMed] [Google Scholar]
- 9.Levesque L E, Brophy J M, Zhang B. The risk for myocardial infarction with cyclooxygenase‐2 inhibitors: a population study of elderly adults. Ann Intern Med 20051421–45. [DOI] [PubMed] [Google Scholar]
- 10.Graham D J, Campen D, Hui R.et al Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo‐oxygenase 2 selective and non‐selective non‐steroidal anti‐inflammatory drugs: nested case‐control study. Lancet 2005365475–481. [DOI] [PubMed] [Google Scholar]
- 11.Kimmel S E, Berlin J A, Reilly M.et al Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med 2005142157–164. [DOI] [PubMed] [Google Scholar]
- 12.Silverstein F E, Faich G, Goldstein J L.et al Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study. JAMA 20002841247–1255. [DOI] [PubMed] [Google Scholar]
- 13.The PreSAP Trial 2005. http://www.fda.gov/ohrms/dockets/ac/05/slides/2005‐4090s1.htm (accessed 17 Oct 2006)
- 14.FDA Joint Meeting with the Drug Safety and Risk Management Advisory Committee 2005. http://www.fda.gov/ohrms/dockets/ac/cder05.html#DrugSafetyRiskMgmt (accessed 17 Oct 2006)
- 15.Levy A R, Tamblyn R M, Fitchett D.et al Coding accuracy of hospital discharge data for elderly survivors of myocardial infarction. Can J Cardiol 1999151277–1282. [PubMed] [Google Scholar]
- 16.Tamblyn R, Lavoie G, Petrella L.et al The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol 199548999–1009. [DOI] [PubMed] [Google Scholar]
- 17.Breslow N E, Day N E. Fitting models to continuous data. In: Breslow NE, Day NE, eds. Statistical methods in cancer research. Vol 2: the design and analysis of cohort studies IARC Scientific Publications number 82. Lyon, France: International Agency of Research on Cancer, 1987178–229.
- 18.Suissa S. Novel approaches to pharmacoepidemiology study design and statistical analysis. In: Strom BL, ed. Pharmacoepidemiology. 3rd edn. Chichester, UK: John Wiley, 2000785–805.
- 19.Hosmer D W, Lemeshow S.Applied logistic regression. New York: John Wiley, 1989
- 20.Collet D. Modelling data from epidemiological studies. In: Collet D, ed. Modelling binary data. New York: Chapman & Hall, 1999223–276.
- 21.Von Korff M, Wagner E H, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol 199245197–203. [DOI] [PubMed] [Google Scholar]
- 22.Schneeweiss S, Seeger J D, Maclure M.et al Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001154854–864. [DOI] [PubMed] [Google Scholar]
- 23.Charlson M E, Pompei P, Ales K L.et al A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 198740373–383. [DOI] [PubMed] [Google Scholar]
- 24.Warner T D, Giuliano F, Vojnovic I.et al Nonsteroid drug selectivities for cyclo‐oxygenase‐1 rather than cyclo‐oxygenase‐2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA 1999967563–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anon Pfizer to finance $100 million Safety Study of Celebrex. The New York Times 14 Dec 2005