Abstract
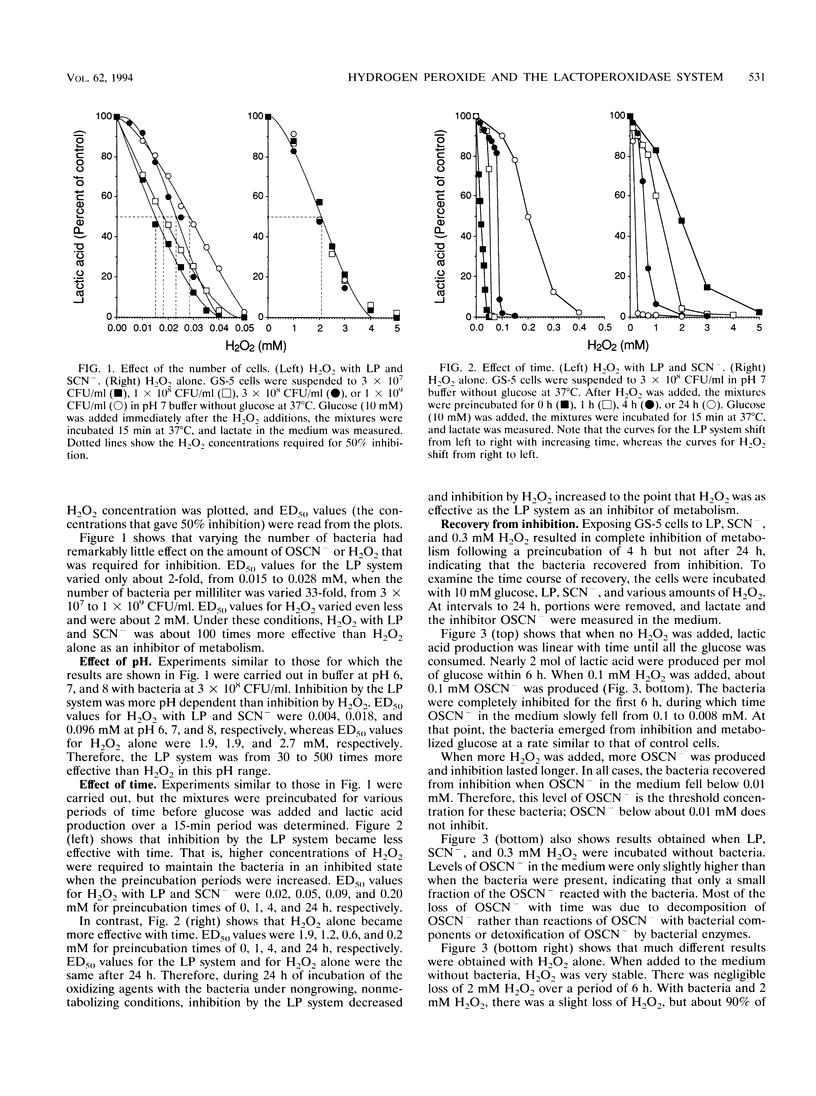
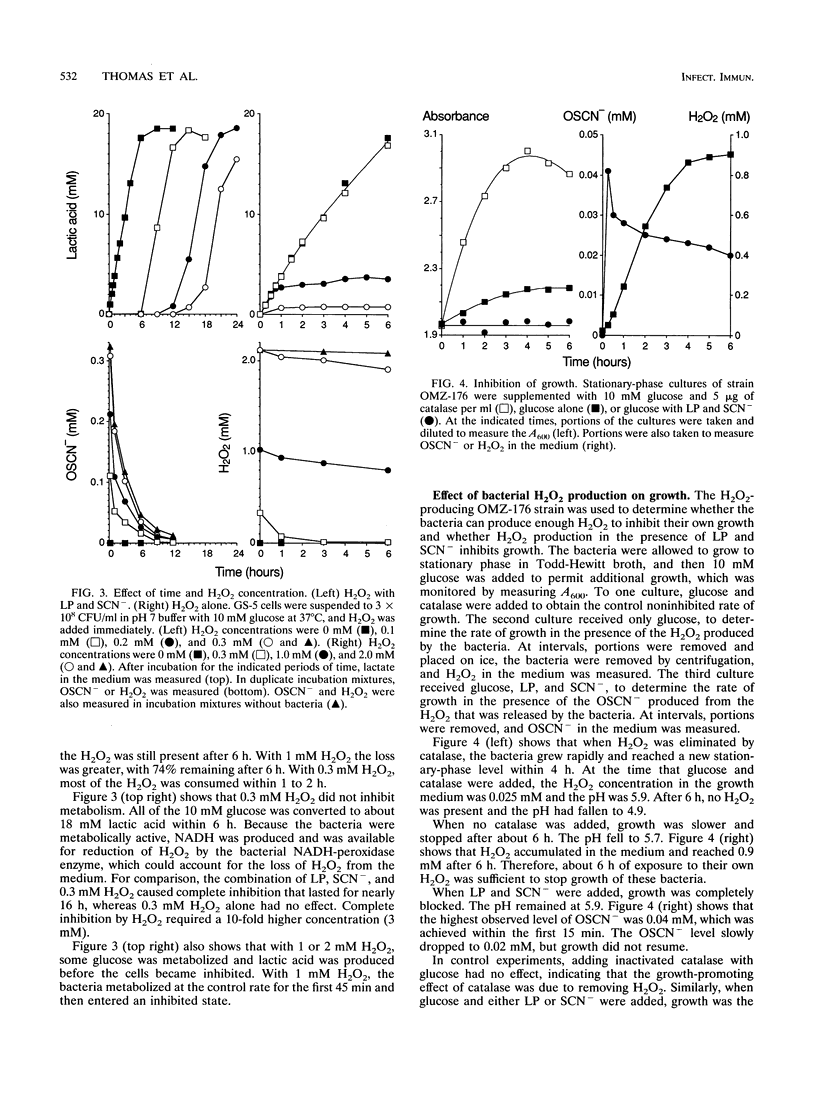
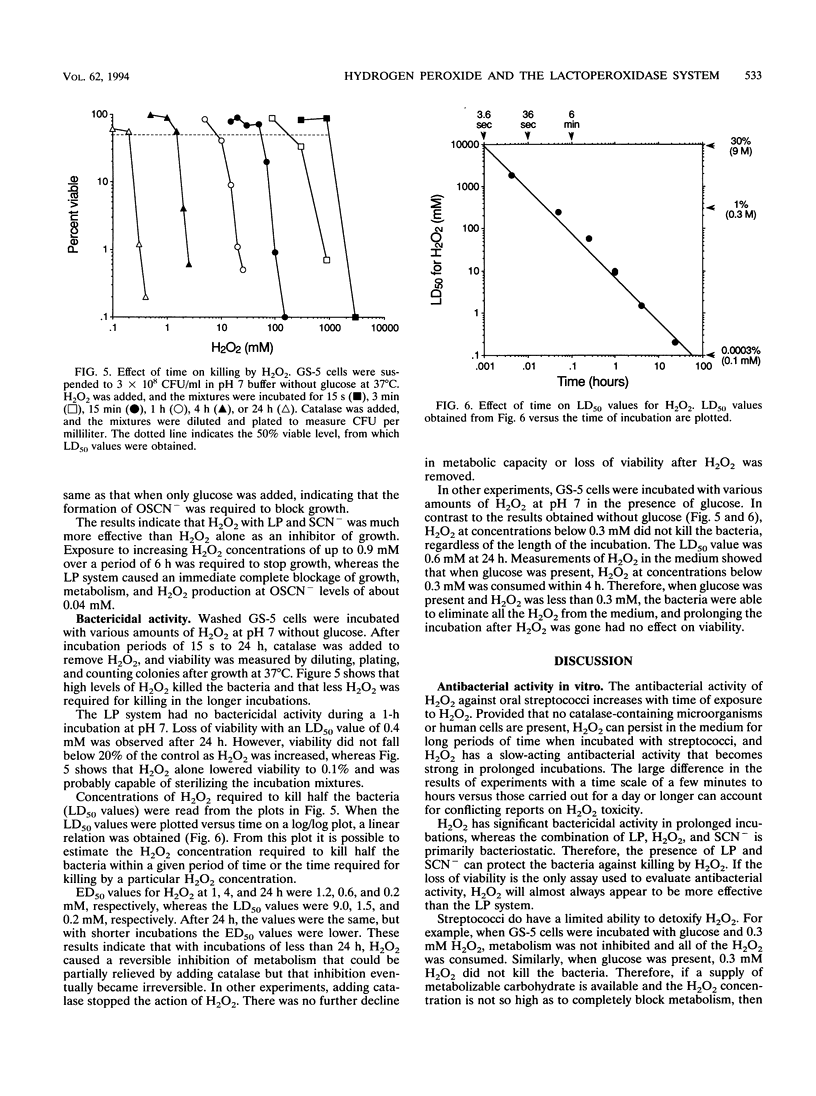
In secreted fluids, the enzyme lactoperoxidase (LP) catalyzes the oxidation of thiocyanate ion (SCN-) by hydrogen peroxide (H2O2), producing the weak oxidizing agent hypothiocyanite (OSCN-), which has bacteriostatic activity. However, H2O2 has antibacterial activity in the absence of LP and thiocyanate (SCN-). Therefore, LP may increase antibacterial activity by using H2O2 to produce a more effective inhibitor of bacterial metabolism and growth, or LP may protect bacteria against the toxicity of H2O2 by converting H2O2 to a less-potent oxidizing agent. To clarify the role of LP, the antibacterial activities of H2O2 and the LP-H2O2-SCN- system were compared by measuring loss of viability and inhibition of bacterial metabolism and growth. The relative toxicity of H2O2 and the LP system to oral streptococci was found to depend on the length of time that the bacteria were exposed to the agents. During incubations of up to 4 h, the LP system was from 10 to 500 times more effective than H2O2 as an inhibitor of glucose metabolism, lactic acid production, and growth. However, if no more H2O2 was added, the concentration of the inhibitor OSCN- fell because of slow decomposition of OSCN-, and when OSCN- fell below 0.01 mM, the bacteria resumed metabolism and growth. In contrast, the activity of H2O2 increased with time. H2O2 persisted in the medium for long periods of time because H2O2 reacted slowly with the bacteria and streptococci lack the enzyme catalase, which converts H2O2 to oxygen and water. After 24 h of exposure, H2O2 was as effective as the LP system as an inhibitor of metabolism. H2O2 also caused a time-dependent loss of viability, whereas the LP system had little bactericidal activity. The concentration of H2O2 required to kill half the bacteria within 15 s was 1.8 M (6%) but fell to 0.3 M (1%) at 2 min, to 10 mM (0.03%) at 1 h, and to 0.2 mM (0.0007%) with a 24-h exposure. The results indicate that if high levels of H2O2 can be sustained for long periods of time, H2O2 is an effective bactericidal agent, and the presence of LP and SCN- protects streptococci against killing by H2O2. Nevertheless, the combination of LP, H2O2, and SCN- is much more effective than H2O2 alone as an inhibitor of bacterial metabolism and growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson M., Carlsson J. Lactoperoxidase and thiocyanate protect bacteria from hydrogen peroxide. Infect Immun. 1982 Jan;35(1):20–24. doi: 10.1128/iai.35.1.20-24.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977 Oct 17;80(1):209–214. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Iwami Y., Yamada T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun. 1983 Apr;40(1):70–80. doi: 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin M. I. Reduced diphosphopyridine nucleotide peroxidase. Intermediates formed on reduction of the enzyme with dithionite or reduced diphosphopyridine nucleotide. J Biol Chem. 1975 Jan 10;250(1):310–317. [PubMed] [Google Scholar]
- Donoghue H. D., Hudson D. E., Perrons C. J. Effect of the lactoperoxidase system on streptococcal acid production and growth. J Dent Res. 1987 Feb;66(2):616–618. doi: 10.1177/00220345870660024701. [DOI] [PubMed] [Google Scholar]
- HOSKINS D. D., WHITELEY H. R., MACKLER B. The reduced diphosphopyridine nucleotide oxidase of Streptococcus faecalis: purification and properties. J Biol Chem. 1962 Aug;237:2647–2651. [PubMed] [Google Scholar]
- Lamberts B. L., Pruitt K. M., Pederson E. D., Golding M. P. Comparison of salivary peroxidase system components in caries-free and caries-active naval recruits. Caries Res. 1984;18(6):488–494. doi: 10.1159/000260809. [DOI] [PubMed] [Google Scholar]
- Mandel I. D., Behrman J., Levy R., Weinstein D. The salivary lactoperoxidase system in caries-resistant and -susceptible adults. J Dent Res. 1983 Aug;62(8):922–925. doi: 10.1177/00220345830620081501. [DOI] [PubMed] [Google Scholar]
- Mansson-Rahemtulla B., Pruitt K. M., Tenovuo J., Le T. M. A mouthrinse which optimizes in vivo generation of hypothiocyanite. J Dent Res. 1983 Oct;62(10):1062–1066. doi: 10.1177/00220345830620101101. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The oxidation of thiocyanate and the nature of the inhibitory compound. Biochem J. 1966 Aug;100(2):382–388. doi: 10.1042/bj1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K. M., Mansson-Rahemtulla B., Tenovuo J. Detection of the hypothiocyanite (OSCN-) ion in human parotid saliva and the effect of pH on OSCN- generation in the salivary peroxidase antimicrobial system. Arch Oral Biol. 1983;28(6):517–525. doi: 10.1016/0003-9969(83)90184-x. [DOI] [PubMed] [Google Scholar]
- Pruitt K. M., Tenovuo J., Fleming W., Adamson M. Limiting factors for the generation of hypothiocyanite ion, an antimicrobial agent, in human saliva. Caries Res. 1982;16(4):315–323. doi: 10.1159/000260614. [DOI] [PubMed] [Google Scholar]
- Pugh S. Y., Knowles C. J. Growth of Streptococcus faecalis var. zymogenes on glycerol: the effect of aerobic and anaerobic growth in the presence and absence of haematin on enzyme synthesis. J Gen Microbiol. 1982 May;128(5):1009–1017. doi: 10.1099/00221287-128-5-1009. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Mäkinen K. K. Concentration of thiocyanate and ionizable iodine in saliva of smokers and nonsmokers. J Dent Res. 1976 Jul-Aug;55(4):661–663. doi: 10.1177/00220345760550042001. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Pruitt K. M., Thomas E. L. Peroxidase antimicrobial system of human saliva: hypothiocyanite levels in resting and stimulated saliva. J Dent Res. 1982 Aug;61(8):982–985. doi: 10.1177/00220345820610081301. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978 May;20(2):456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Bates K. P., Jefferson M. M. Hypothiocyanite ion: detection of the antimicrobial agent in human saliva. J Dent Res. 1980 Sep;59(9):1466–1472. doi: 10.1177/00220345800590090201. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Bates K. P., Jefferson M. M. Peroxidase antimicrobial system of human saliva: requirements for accumulation of hypothiocyanite. J Dent Res. 1981 Apr;60(4):785–796. doi: 10.1177/00220345810600040401. [DOI] [PubMed] [Google Scholar]
- Thomas E. L. Disulfide reduction and sulfhydryl uptake by Streptococcus mutans. J Bacteriol. 1984 Jan;157(1):240–246. doi: 10.1128/jb.157.1.240-246.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Fishman M. Oxidation of chloride and thiocyanate by isolated leukocytes. J Biol Chem. 1986 Jul 25;261(21):9694–9702. [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Jefferson M. M. Preparation and characterization of chloramines. Methods Enzymol. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- Thomas E. L. Lactoperoxidase-catalyzed oxidation of thiocyanate: equilibria between oxidized forms of thiocyanate. Biochemistry. 1981 May 26;20(11):3273–3280. doi: 10.1021/bi00514a045. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A. Oxygen metabolism of Streptococcus mutans: uptake of oxygen and release of superoxide and hydrogen peroxide. J Bacteriol. 1983 Jun;154(3):1236–1244. doi: 10.1128/jb.154.3.1236-1244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A., Smith K. W., Chwang A. K. Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system. Infect Immun. 1983 Feb;39(2):767–778. doi: 10.1128/iai.39.2.767-778.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



