Abstract
Nearly half of patients with symptoms of heart failure are found to have a normal left ventricular (LV) ejection fraction. This has variously been labelled as diastolic heart failure, heart failure with preserved LV function or heart failure with a normal ejection fraction (HFNEF). As recent studies have shown that systolic function is not entirely normal in these patients, HFNEF is the preferred term. The epidemiology, aetiology and possible pathophysiology of this contentious condition are reviewed. The importance of the remodelling process in determining whether a patient presents with systolic heart failure or HFNEF is emphasised and this can be used to classify patients in a more rational manner.
It has been realised for some time that many patients presenting with symptoms of what is apparently heart failure are found on further investigation to have a normal left ventricular ejection fraction (LVEF). This has been variously labelled as diastolic heart failure, heart failure with preserved systolic function or heart failure with a normal ejection fraction (HFNEF). The preferred term should be HFNEF because accumulative evidence suggests that the physiological abnormalities in these patients are not restricted to diastole only, and systolic function is not entirely “preserved” when measures other than the ejection fraction are used. There are epidemiological, clinical, pathological and physiological similarities and differences between patients who have reduced LVEF with ventricular dilatation, what is commonly called systolic heart failure (SHF), and those with HFNEF.
EPIDEMIOLOGY
Population‐based epidemiology prevalence studies suggest that nearly half the patients with heart failure have HFNEF, although in hospital cohorts the percentage appears to be less. The proportion of patients with HFNEF in the various studies ranges from 40–71% (with a mean of 56%).1 However, all these studies are compromised by variable definitions of heart failure and the precise threshold for what is considered to be a normal LVEF. In the Cardiovascular Health Study 80% of patients had an LVEF > 0.45 but only 55% had an LVEF > 0.55.2 In hospital‐based cohort studies the proportion of patients with HFNEF is slightly lower, ranging from 24–55% (mean 41%).1 A possible explanation is that patients with HFNEF have less severe symptoms or are less frequently admitted. Patients with HFNEF tend to be older on average than those with SHF and in most studies the majority have been women. This appears to be a consistent feature. HFNEF appears to be common in China and India perhaps because of the high prevalence of hypertension in these communities.3 The ageing of the populations in Asia combined with hypertension that is often poorly treated presage a substantial increase in the number of patients with HFNEF in these areas.4 The morbidity, hospitalisation rates and healthcare costs per patient, however, are very similar between patients with HFNEF and those with SHF.1 Reported mortality varies widely. In the Framingham Heart Study, for patients with HFNEF the annual mortality was 8.7% compared with 3% in matched controls and for SHF was 18.9% compared with a 4.1% in age‐ and sex‐matched controls over 6.2 years.5 In contrast, in the Cardiovascular Health Study the population‐attributable mortality risk was greater for those with HFNEF than those with SHF6 but this is partly explained by the higher prevalence of HFNEF in the elderly population.
Many of these epidemiology studies may be unreliable because at the moment HFNEF is a diagnosis of exclusion, as the criteria for diastolic dysfunction independent of age‐related changes have not been clearly delineated (see below). Many patients with obesity, chronic obstructive airways disease or ankle oedema are often said to have heart failure. Studies are needed that carefully assess through metabolic exercise testing whether these patients indeed have true heart failure. In a revealing study Caruana et al7 found that a third of their patients thought to have HFNEF were either obese or very obese, half had considerable reductions in respiratory function (forced expiratory volume in 1 s ⩽ 70%) and many had evidence of a myocardial infarction or ischaemia. Only seven of 109 patients with a diagnosis of HFNEF did not have another explanation for their symptoms. However, in this study only simple mitral inflow E:A ratios were used to establish diastolic dysfunction. Many of these elderly patients do have several co‐morbidities that often contribute to their symptoms.
AETIOLOGY AND PATHOPHYSIOLOGY
A history of hypertension with left ventricular (LV) hypertrophy is commonly associated with HFNEF.1 Also, new‐onset atrial fibrillation (AF) is common, and the loss of the atrial contribution and reduced filling time may combine to precipitate pulmonary oedema.1 Hypertension predisposes towards the development of AF especially if LV filling pressures are high and left atrial size is increased. Thus, the onset of AF in a patient with hypertension may be the precipitating factor for the symptoms of heart failure to develop and the subsequent hospital admission. Ischaemia and diabetes are also important. In a study from Hong Kong it was clear that hypertension, ischaemic heart disease and diabetes overlapped and all were common in patients with HFNEF.3 All of these aetiological factors can impair both systolic and diastolic function, particularly ventricular long axis function, even in the presence of a normal LVEF.8,9
The development of newer echocardiographic techniques such as tissue Doppler imaging has enabled a more accurate assessment of ventricular function. In an early study Yip et al10 showed that both peak annular systolic and peak early diastolic velocities and the respective excursions that are measures of ventricular long axis function were lower in patients with HFNEF than in age‐matched controls. These findings have now been confirmed in six other studies.11,12,13,14,15,16 Thus, despite a normal ejection fraction, systolic function in the long axis is not normal in HFNEF. This should come as no surprise, as both LV hypertrophy and fibrosis clearly affect systole as much as diastole. Shan et al17 showed that both peak annular systolic velocity and early diastolic velocity are equally affected by interstitial fibrosis within the myocardium. Physiologically, systole and diastole are closely intertwined. We found a close relationship between annular systolic and diastolic velocities across a wide range of LVEFs,18 which has been confirmed by others.13,16 In reality systole and diastole constitute one cycle, and the major determinant of early diastolic filling is the strength and coordination of the previous systole, which is the driver for ventricular suction. In addition, incoordinate systolic contraction prolongs isovolumic relaxation and further impairs diastolic function.19
Interestingly, peak early diastolic velocity has emerged as a powerful predictor of prognosis in a variety of cardiac diseases including heart failure.20 This may be because this measurement of motion of the ventricular base during early diastole reflects both systolic and diastolic function of the ventricle, because early diastolic filling is so dependent on LV suction. Moreover, the subendocardial fibres, which are mainly responsible for long axis contraction, may be more susceptible to the effects of fibrosis, hypertrophy and ischaemia because of their position, and thus explain why this measurement is a good early marker of disease. In addition, hypertension, LV hypertrophy, ageing and diabetes all alter global myocardial architecture and fibre orientation, which would probably have important effects on ventricular torsion and recoil during relaxation. Reduced ventricular twist and long axis motion during systole also affect ventricular suction.21
It is thus artificial to separate the two phases of the cardiac cycle. Despite this, some have argued that in HFNEF systolic function is completely normal, and that the clinical condition is due entirely to diastolic dysfunction alone, and SHF and HFNEF are distinctly different.22 These studies are based on global measurements derived from pressure–volume relationships; these take no account of regional dysfunction or abnormalities of long axis function, which are compensated for initially by increased radial function.13 Even measures such as tau and LV end diastolic pressure–volume relationships have considerable theoretical and practical drawbacks: neither accurately measures “relaxation” or “stiffness” as popularly supposed.23 Global pressure–volume loops can be remain normal despite significant changes in myocardial architecture and shape, which perhaps are reflected better by the long axis measurements.
Titin, a giant sarcomere protein that acts like a molecular spring, may also have a role, as titin isoform shifting may have an impact on diastolic function. In idiopathic dilated cardiomyopathy, Nagueh et al24 have recently shown an increase in the N2BA:N2B isoform ratio compared with controls. This shift to a larger isoform would predict a substantial decrease in passive myocardial stiffness, which was found in myocardial strips, but also affects the restoring forces and elastic recoil of the cardiac myocyte and hence ventricular suction.
Remodelling
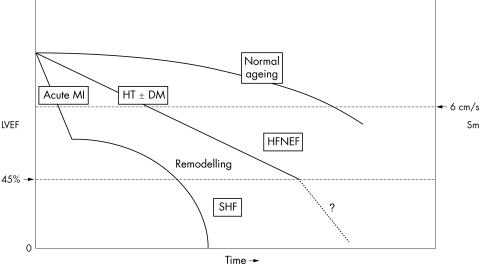
The main physiological difference between SHF and HFNEF is the increase in ventricular volume and change in shape due to ventricular remodelling. A myocardial infarction (or rarely viral myocarditis) appears to be a potent stimulant for the remodelling process, which leads to increased ventricular volumes and reduced ejection fraction.25 In hypertensive heart disease remodelling is a slower process. Initially LV hypertrophy by itself leads to reduced systolic and diastolic function particularly in the long axis.8 Compensatory increased radial contraction normalises the ejection fraction. However, at later stages further remodelling will occur, the LV volumes will increase and the patient will slip from HFNEF to more obvious SHF (fig 1). Thus, from a physiological point it is more sensible to categorise patients with heart failure according to whether remodelling has taken place. Remodelling is a very important therapeutic target and reversing remodelling is probably a powerful predictor of improvement. Nearly all treatments that are proven to reduce mortality and improve symptoms in heart failure have also induced reverse remodelling—for example, β blockers and cardiac resynchronisation therapy.26,27
Figure 1 Time course and pattern of development of heart failure primarily caused by myocardial infarction (MI), with pronounced remodelling and shape change leading to systolic heart failure (SHF), and of heart failure primarily caused by hypertension (HT), with or without diabetes mellitus (DM), leading to heart failure with a normal ejection fraction (HFNEF). Lower normal limits of left ventricular ejection fraction (LVEF) and peak annular systolic velocity (Sm) are indicated on the axes. Both patients with an MI and patients with HT may pass through an HFNEF and an SHF period.
Peripheral factors
In a recent experimental study of HFNEF the time to complete relaxation was significantly longer than in controls, which worsened with increased arterial pressure.28 Also, end systolic elastance was increased in this experimental heart failure model and was closely linked to collagen volume fraction. Afterload affects both systolic and diastolic LV performance, prolonging contraction and relaxation. This effect is seen early in the progression of systolic dysfunction and leads to a shortening of the diastolic filling period. This action of an increased afterload would be particularly troublesome with faster heart rates such as with exercise or AF. Kawaguchi et al29 found in humans that end systolic elastance (stiffness) was higher in patients with HFNEF as was effective arterial elastance due to reduced total arterial compliance, and these were higher than that associated with ageing or hypertension. This ventricular–arterial stiffening, presumably due to abnormal myocardial and arterial collagen, amplifies stress‐induced hypertension, thus worsening diastolic dysfunction. Impaired renal function and renal arterial atherosclerosis in the elderly may also be involved in causing rapid rises in blood pressure and excessive fluid retention.
CLINICAL FEATURES AND DIAGNOSIS
Although there are clinical differences between the typical patient presenting with HFNEF and with SHF these relate more to aetiology and whether remodelling has taken place (table 1). The typical patient with HFNEF is an elderly women with a history of hypertension often with diabetes whose heart failure is episodic, often precipitated by an episode of AF, ischaemia or infection.30 In fact these simple criteria based on aetiology and the presence or absence of ventricular remodelling point to a more useful classification. Patients with HFNEF usually have hypertensive heart failure with LV hypertrophy, whereas the typical SHF patient has usually had a previous myocardial infarction with significant LV remodelling, myocarditis or idiopathic dilated cardiomyopathy. Approaching the diagnosis of all types of heart failure along the following lines appears more useful. Firstly, establish the presence of heart failure by symptoms and concentrations of brain natriuretic peptide (and exercise testing if unsure). Secondly, determine the main aetiology and mechanisms: hypertension or ischaemia, infarction, etc. Thirdly, determine whether remodelling has taken place (are LV volumes increased)? Lastly, look for the presence of additional deleterious factors: dyssynchrony, arrhythmias, metabolic/electrolyte abnormalities, etc.
Table 1 Comparison of clinical features of HF with reduced and normal EF.
| HF with reduced EF (SHF) | HF with normal EF | |
|---|---|---|
| Sex | More men than women | More women than men |
| Age (years) | 50–60 | 60–70 |
| Aetiology | MI; idiopathic DCM | HT ± DM; AF; transient ischaemia |
| Clinical progress | Persistent HF | Often episodic HF |
| Ventricular remodelling (increased LV volumes) | +++ | 0 |
| LV hypertrophy | +/− | +++ |
| Dyssynchrony | Common | Possibly less common |
| Mitral inflow pattern | RFP or ARP | ARP |
| Peak mitral annular systolic velocity | Greatly reduced | Moderately reduced |
| Peak mitral annular early diastolic velocity | Greatly reduced | Moderately reduced |
| LA pressure | Raised | Raised |
| LA volume | Raised | Raised |
AF, atrial fibrillation; ARP, abnormal relaxation pattern; DCM, dilated cardiomyopathy; EF, ejection fraction; HF, heart failure; HT ± DM, hypertension with or without diabetes mellitus; LA, left atrial; LV, left ventricular; MI, myocardial infarction; RFP, restrictive filling pattern; SHF, systolic heart failure.
This process focuses on the two major stages of the clinical process: firstly, deciding whether this is heart failure and, secondly, identifying treatable factors such as ischaemia, remodelling, dyssynchrony, etc. Echocardiography has a vital role in all these processes. Measurement of the LVEF is not relevant. Clearly, the theoretical underpinning of the concept that SHF and HFNEF are physiologically fundamentally different has been undermined by recent research as outlined above. Both conditions have a mixture of systolic and diastolic abnormalities and it appears more useful to classify according to the aetiology and the mechanisms involved in the individual patient, which may be different. Measurements of long axis function are sensitive and can be used to confirm the presence of impaired systolic and diastolic dysfunction, and peak early diastolic velocity is a powerful predictor of future prognosis.20 However, all measurements of long axis function and mitral inflow velocities need to be corrected for age. Ageing has a powerful deleterious effect on ventricular function and on these diastolic indices. Criteria for diagnosis of HFNEF based on diastolic measurements of mitral inflow velocities are not usually corrected for age. Indeed the whole definition of diastolic dysfunction, based on echocardiography, is difficult and there is no ideal method. Extreme mitral filling patterns such as the restrictive filling pattern are obvious indicators of severe diastolic dysfunction but usually occur only in the presence of severe systolic dysfunction as well. Indeed, Sim et al31 found no difference in the LV filling patterns seen on echocardiography between an appropriate reference population and patients with breathlessness. The previous guidelines on diagnosis of “diastolic heart failure” are now less relevant in view of these recent findings and new guidelines are clearly required.
TREATMENT
There is little evidence to guide treatment, as previously patients with HFNEF have been excluded from clinical trials on the basis of a normal LVEF. The CHARM (Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity) ‐Preserved trial assessed the additional effect of treatment with candesartan on cardiovascular death or admissions to hospital.32 Candesartan had a modest impact in preventing hospital admissions but no effect on cardiovascular death. In one study diuretic withdrawal was associated with more frequent but non‐significant recurrence of heart failure in patients with HFNEF.33 Digoxin reduced hospitalisations in another, although it had no effect on mortality.34 Ongoing studies may provide more data for a more evidence‐based approach. At the moment diuretics can be recommended to reduce symptoms of breathlessness. On the basis that many of these patients have LV hypertrophy, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers may be of value. Clinical trials in this group of patients are often difficult because many patients with HFNEF are elderly and they have much other co‐morbidity, in particular renal failure.
CONCLUSION
HFNEF is a relatively common cause of heart failure symptoms. As in all forms of heart failure there is a mixture of abnormalities of systolic and diastolic function. LVEF is not a good measurement on which to base a classification that dichotomises patients with heart failure into two groups. Aetiology and the presence of remodelling (increased ventricular volumes) are clinically more useful parameters to use for a classification. It is still unclear how often HFNEF evolves into SHF due to ventricular remodelling but this probably does occur. However, HFNEF is probably overdiagnosed because of the absence of good age‐independent measurements of diastolic dysfunction, and many patients thought to have HFNEF may not have heart failure but merely mild fluid overload.
ACKNOWLEDGEMENTS
I thank Drs Gabriel Yip, Alan Fraser, Derek Gibson, Michael Frenneaux, Mei Wang and Cheuk‐Man Yu for their helpful discussions and thoughts over the past few years.
Abbreviations
AF - atrial fibrillation
CHARM - Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity
HFNEF - heart failure with a normal ejection fraction
LV - left ventricular
LVEF - left ventricular ejection fraction
SHF - systolic heart failure
Footnotes
Competing interests: None declared.
References
- 1.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function: epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 200443317–327. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman D W, Gardin J M, Gottdiener J S.et al Importance of heart failure with preserved systolic function in patients >or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 200187413–419. [DOI] [PubMed] [Google Scholar]
- 3.Yip G W K, Ho P P Y, Woo K S.et al Comparison of frequencies of left ventricular systolic and diastolic heart failure in Chinese living in Hong Kong. Am J Cardiol 199984563–567. [DOI] [PubMed] [Google Scholar]
- 4.Sanderson J E, Tse T F. Heart failure: a global disease requiring a global response. Heart 200389585–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasan R S, Larson M G, Benjamin E J.et al Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population‐based cohort. J Am Coll Cardiol 1999331948–1955. [DOI] [PubMed] [Google Scholar]
- 6.Gottdiener J S, McClelland R L, Marshall R.et al Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002137631–639. [DOI] [PubMed] [Google Scholar]
- 7.Caruana L, Petrie M C, Davie A P.et al Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart failure” or from misdiagnosis? A prospective descriptive study. BMJ 2000321215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Yip G W K, Wang A.et al Tissue Doppler imaging provides incremental prognostic value in patients with hypertension and left ventricular hypertrophy. J Hypertens 200523183–191. [DOI] [PubMed] [Google Scholar]
- 9.Fang Z Y, Leano R, Marwick T H. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci (Lond) 200410653–60. [DOI] [PubMed] [Google Scholar]
- 10.Yip G, Wang M, Zhang Y.et al Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart 200287121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrie M C, Caruana L, Berry C.et al “Diastolic heart failure” or heart failure caused by subtle left ventricular systolic dysfunction. Heart 20028729–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikitin N P, Witte K K, Clark A L.et al Color tissue Doppler‐derived long‐axis left ventricular function in heart failure with preserved global systolic function. Am J Cardiol 2002901174–1177. [DOI] [PubMed] [Google Scholar]
- 13.Vinereanu D, Nicolaides E, Tweddel A C.et al “Pure” diastolic dysfunction is associated with long‐axis systolic dysfunction: implications for the diagnosis and classification of heart failure. Eur J Heart Fail 20057820–828. [DOI] [PubMed] [Google Scholar]
- 14.Garcia E H, Perna E R, Farias E F.et al Reduced systolic performance by tissue Doppler in patients with preserved and abnormal ejection fraction: new insights in chronic heart failure. Int J Cardiol 2006108181–188. [DOI] [PubMed] [Google Scholar]
- 15.Yu C M, Lin H, Yang H.et al Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation 20021051195–1201. [DOI] [PubMed] [Google Scholar]
- 16.Bruch C, Gradaus R, Gunia S.et al Doppler tissue analysis of mitral annular velocities: evidence for systolic abnormalities in patients with diastolic heart failure. J Am Soc Echocardiogr 2003161031–1036. [DOI] [PubMed] [Google Scholar]
- 17.Shan K, Bick R J, Poindexter B J.et al Relation of tissue Doppler derived myocardial velocities to myocardial structure and beta‐adrenergic receptor density in humans. J Am Coll Cardiol 200036891–896. [DOI] [PubMed] [Google Scholar]
- 18.Yip G W, Zhang Y, Tan P Y H.et al Left ventricular long‐axis changes in early diastole and systole: impact of systolic function on diastole. Clin Sci (Lond) 2002102515–522. [PubMed] [Google Scholar]
- 19.Gibson D G, Brown D J. Relation between diastolic left ventricular wall stress and strain in man. Br Heart J 1974361066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Yip G W K, Yu C M.et al Independent and incremental prognostic value of early diastolic annular velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol 200545272–277. [DOI] [PubMed] [Google Scholar]
- 21.Ashikaga H, Criscione J C, Omens J H.et al Transmural left ventricular mechanics underlying torsional recoil during relaxation. Am J Physiol Heart Circ Physiol 2004286H640–H647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baicu C F, Zile M R, Aurigemma G P.et al Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation 20051112306–2312. [DOI] [PubMed] [Google Scholar]
- 23.Gibson D G, Francis D P. Clinical assessment of left ventricular diastolic function. Heart 200389231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagueh S F, Shah G, Wu Y.et al Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation 2004110155–162. [DOI] [PubMed] [Google Scholar]
- 25.Mann D L, Bristow M R. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 20051112837–2849. [DOI] [PubMed] [Google Scholar]
- 26.Groenning B A, Nilsson J C, Sondergaard L.et al Antiremodeling effects on the left ventricle during beta‐blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol 2000362072–2080. [DOI] [PubMed] [Google Scholar]
- 27.Yu C M, Chau E, Sanderson J E.et al Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 2002105438–445. [DOI] [PubMed] [Google Scholar]
- 28.Munagala V K, Hart C Y T, Burnett J C.et al Ventricular structure and function in aged dogs with renal hypertension: a model of experimental diastolic heart failure. Circulation 20051111128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaguchi M, Hay I, Fetics B.et al Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 2003107714–720. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee P, Clark A L, Nikitin N.et al Diastolic heart failure: paroxysmal or chronic? Eur J Heart Fail 20046427–431. [DOI] [PubMed] [Google Scholar]
- 31.Sim M F, Ho S F, O'Mahoney M S.et al European reference values for Doppler indices of left ventricular diastolic filling. Eur J Heart Fail 20046433–438. [DOI] [PubMed] [Google Scholar]
- 32.Yusuf S, Pfeffer M A, Swedberg K, for the CHARM investigators and Committees et al Effects of candesartan in patients with chronic heart failure and preserved left ventricular ejection fraction: the CHARM‐Preserved trial. Lancet 2003362777–781. [DOI] [PubMed] [Google Scholar]
- 33.Van Kraaij D J, Jansen R W, Sweep F C.et al Neurohormonal effects of furosemide withdrawal in elderly heart failure patients with normal systolic function. Eur J Heart Fail 2003547–53. [DOI] [PubMed] [Google Scholar]
- 34.Digitalis Intervention Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997336525–533. [DOI] [PubMed] [Google Scholar]