Abstract
Background
Various studies have reported a close correlation between real‐time three‐dimensional echocardiography (RT3DE) and cine magnetic resonance imaging studies for the assessment of cardiac volumes and mass.
Objective
The aim of our study was to evaluate changes in left ventricular volumes and mass in subjects with different pathophysiological conditions. A ratio between left ventricular mass and end‐diastolic volume (LVRI), detected by RT3DE, was used to describe various patterns of left ventricular remodelling.
Methods
RT3DE was performed to calculate left ventricular end‐diastolic (LVEDV) and end‐systolic volume (LVESV), ejection fraction (LVEF) and mass in 220 selected subjects. Of these, 152 were healthy volunteers, 19 top‐level rowers, 23 patients with dilated cardiomyopathy and 26 patients with hypertrophic cardiomyopathy. Off‐line analysis was performed by two independent operators by tracing manual endocardial and epicardial borders of the left ventricle through eight cutting planes. Inter‐ and intra‐observer variability were calculated.
Results
Despite the increase in LV volume and mass in the rowers, LVRI remained unchanged compared with control subjects (p = 0.455), while significantly lower values were found patients with dilated cardiomyopathy (p<0.001) and significantly higher values in patients with hypertrophic cardiomyopathy (p<0.001). There was inter‐ and intra‐observer variability.
Conclusion
The LVRI may serve as a simple and useful indicator of left ventricular adaptation to physiological and pathological conditions.
Keywords: 3D echocardiography, LV mass, LV remodelling, LV volumes
Left ventricular (LV) remodelling may be defined as a modification in shape, size, and function of the left ventricle due to physiological or pathological conditions.1,2 For example, an adaptation to increased haemodynamic overload induced by chronic and intensive exercise has been extensively described and reported as “athlete's heart”.3,4 In contrast, pathological changes can be seen in different primary and secondary disorders of the ventricles due to ischaemic cardiomyopathy, hypertension, valvular heart disease, and hypertrophic and dilated cardiomyopathy.5,6,7,8,9
LV remodelling is a result of many disorders that include changes not only in LV cavity size but also in LV volume. While the importance of LV remodelling is well recognised, most clinical studies and trials examining remodelling rely primarily on changes in LV diameter or cavity area, or, in a few studies, LV volumes measured by either two‐dimensional (2D) echocardiography or radionuclide methods.10,11,12,13,14 Few clinical investigations have focused on a combination of LV volume and mass. For many years, morphological and volumetric assessment of the left ventricle has been based on 2D echocardiography. However, this approach has some limitations, principally due to the use of geometric assumptions for deriving volumetric parameters, a high interobserver variability and a probe positioning bias. The ideal imaging technique for the assessment of serial ventricular volumes should be widely available, accurate and reproducible. Real‐time three‐dimensional (3D) echocardiography can meet these criteria.15,16,17,18,19
In the present study, we have studied left ventricular volume, mass and ejection fraction changes in a heterogeneous population including healthy volunteers, athletes with hyperphysiological adaptation to continuous intensive training, and patients with pathological conditions such as dilated and hypertrophic cardiomyopathy. We propose a new index called the left ventricular remodelling index (LVRI) as the ratio between left ventricular mass (LVM) and left ventricular end‐diastolic volume (LVEDV) derived from real‐time 3D data sets.
Methods
The study was carried out at three centres: “La Sapienza” University Hospital, Rome, Italy, the National Institute of Sports Medicine, Rome, Italy and Tufts‐New England Medical Center, Boston, USA. All subjects gave their written informed consent for the study. The study was approved by the research ethics committees of the three centres.
Study population and patient selection
The study population consisted of 224 selected subjects. Of these, 152 were healthy volunteers (V), 19 were top‐level athletes (A), 27 were patients affected by dilated cardiomyopathy (DC) and 26 were patients affected by hypertrophic cardiomyopathy (HC). Selection criteria and classification in each subgroup were based on clinical history, blood pressure, clinical examination, electrocardiogram (ECG) findings and 2D/Doppler echocardiography.
The 152 healthy volunteers (124 males and 28 females) satisfied the following criteria: normal physical examination, normal blood pressure (<135 mm Hg and <80 mm Hg), normal ECG findings, no history of chest pain or dyspnea, no diabetes, and normal 2D echocardiographic and Doppler examination. None of the subjects was on medication. Any potential subjects with evidence of heart disease, hypertension or other systemic disorders were excluded from the study.
The athletes were members of the Italian Olympic Rowing Team, selected on the basis of long‐term exercise conditioning (>3 consecutive years) with a high level of achievement in the World Championships and Olympic Games. All the athletes were periodically screened at the National Institute of Sports Medicine.20,21,22
Within the dilated cardiomyopathy group, 18 patients were diagnosed with ischaemic cardiomyopathy and nine with idiopathic dilated cardiomyopathy. The diagnosis of dilated cardiomyopathy was based on M‐mode and 2D/Doppler echocardiographic examination demonstrating an end‐diastolic diameter >3.2 cm/m2 and a left ventricular ejection fraction (LVEF) <40%.23 The patients with ischaemia had a history of myocardial infarction with ECG evidence of Q waves and documented echocardiographic akinetic/dyskinetic wall‐motion abnormalities. In addition to satisfying the aforementioned echocardiographic criteria, the patients with primary idiopathic dilated cardiomyopathy had had a normal coronary angiography performed within the previous 6 months.
The diagnosis of hypertrophic cardiomyopathy was determined in 26 patients by 2D echocardiography on the basis of a non‐dilated and hypertrophic left ventricle, in the absence of any other cardiac or systemic disease capable of producing wall thickening of similar magnitude.24,25 Of these, seven patients had obstruction; none of the others had >30 mm Hg peak Doppler gradient in the left ventricular outflow tract under basal conditions. Moreover, none of the 26 patients had a qualitative mitral regurgitation of more than 2+/4+.
General exclusion criteria were any of: systemic blood pressure >140 mm Hg, atrial fibrillation, poor echocardiographic acoustic window, or significant mitral or aortic regurgitation (>2+/4+).
Two‐dimensional echocardiography
Two‐dimensional echocardiography was carried out by expert cardiologists using a commercial ultrasound machine (Sonos 7500, Philips, Andover, MA, USA) with an S3 probe (2–4 MHz). A complete examination was carried out on each subject including 2D and Doppler analysis. The assessment of left ventricular wall thickness and internal diameter was performed in the parasternal long‐axis view. The LVEF was estimated in four‐ and two‐chamber windows using the modified Simpson's method.
Three‐dimensional echocardiography
Real‐time three‐dimensional echocardiography (RT3DE) was performed by expert cardiologists using a Sonos 7500 system with Live 3D Echo (Philips) equipped with the X4 transducer. The acquisition time for RT3DE was recorded for multiple full volume data sets from the apical four‐chamber view. Three acquisitions performed in the full‐volume mode were stored for each patient and analysed off‐line with 4D Echo‐View (version 5.2; TomTec, Unterschleissheim, Germany). LVEDV and end‐systolic volume (LVESV), LVEF and LVM were measured by manual tracing of epicardial and endocardial borders through eight different rotational cutting planes obtained through the LV long axis in end‐diastole and end‐systole; papillary muscles were excluded from tracing. LVM was determined by multiplying the volumetric value by the relative density of the myocardium (1.05 g/ml) (fig 1).
Figure 1 Off‐line analysis: endocardial and epicardial border tracing through eight different cutting planes to determine left ventricular end‐diastolic volume (on the left) and mass (on the right).
The LVRI was obtained by calculating the ratio of LVM to LVEDV. The time taken for the off‐line quantitative analysis of the 3D data sets was also recorded.
Statistical analysis
All data are expressed as mean±standard deviation or median values (interquartile range, IQR). Differences in echocardiographic parameters were examined using one‐way analysis of variance (ANOVA) followed by a Scheffe post‐hoc test.
To determine the interobserver variability, all measurements were taken by two observers blinded to the values obtained during the selection process. To assess intra‐observer variability, all measurements were repeated 1 week later by an observer blinded to the results of the previous measurements. Inter‐ and intra‐observer variability were assessed using the inter‐ and intra‐class correlation coefficient (ICC).
Results
Table 1 shows the demographic characteristics, and the 2D and 3D echocardiographic parameters.
Table 1 Demographic characteristics and LV parameters detected by RT3DE in the study population.
| Subjects | Healthy volunteers (V) | Top‐level athletes (A) | p Value V v A | Dilated cardiomyo‐ pathy (DC) | p Value V v DC | p Value DC v A | Hypertrophic cardiomyo‐ pathy (HC) | p Value V v HC | p Value DC v HC | p Value A v HC |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 152 | 19 | 23 | 26 | ||||||
| Age, years | 38 (32–45) | 25 (23–27) | <0.001 | 56 (51–59) | <0.013 | <0.001 | 50 (46–54) | <0.001 | 0.387 | <0.001 |
| Male, n (%) | 105 (69) | 15(79) | 0.438 | 19 (83) | 0.225 | 1.000 | 18 (69) | 1.000 | 0.333 | 0.517 |
| BSA, m2 | 1.88±0.19 | 2.07±0.20 | <0.001 | 1.91±0.18 | 0.478 | 0.009 | 1.96±0.20 | 0.627 | 0.365 | 0.075 |
| 2D echo | ||||||||||
| LVIVS, mm | 9.2±1.4 | 11.8±0.8 | <0.001 | 8.7±0.7 | 0.095 | <0.001 | 17.4±3.3 | <0.001 | <0.001 | <0.001 |
| LVPW, mm | 8.6±1.3 | 11.0±0.6 | <0.001 | 7.7±0.8 | 0.002 | <0.001 | 13.7±1.5 | <0.001 | <0.001 | <0.001 |
| LVEDV, ml | 46.4±7.3 | 55.3±4.9 | <0.001 | 62.8±5.3 | <0.001 | <0.001 | 45.8±3.9 | 0.683 | <0.001 | <0.001 |
| LVESV, ml | 28.8±5.3 | 35.4±8.1 | <0.001 | 48.5±4.1 | <0.001 | <0.001 | 22.1±5.5 | <0.001 | <0.001 | <0.001 |
| VEF, % | 60±7 | 59±6 | 0.552 | 30±8 | <0.001 | <0.001 | 65±5 | <0.001 | <0.001 | <0.001 |
| 3D echo | ||||||||||
| LVEDV, ml | 106.43±22.03 | 196.16±34.45 | <0.001 | 287.06±57.71 | <0.001 | <0.001 | 111.47±25.93 | <0.001 | <0.001 | <0.001 |
| LVEDV, ml/m2 | 56.61±11.72 | 94.76±16.64 | 150.20±30.21 | 56.87±13.23 | ||||||
| LVESV, ml | 40.84±9.56 | 82.79±14.9 | <0.001 | 207.76±52.74 | <0.001 | <0.001 | 43.41±14.95 | <0.001 | <0.001 | <0.001 |
| LVESV, ml/m2 | 21.72±5.09 | 40.00±7.20 | 108.77±27.61 | 22.15±7.63 | ||||||
| LVM, g | 109.62±24.21 | 218.81±34.41 | <0.001 | 157.46±43.23 | <0.001 | <0.001 | 262.09±77.87 | <0.001 | 0.003 | |
| LVM, g/m2 | 58.31±12.88 | 105.71±18.56 | 82.44±22.63 | 133.72±39.73 | ||||||
| LVEF, % | 62±6 | 60±5 | 0.166 | 28±8 | <0.001 | 61±7 | 0.108 | |||
| LVRI | 1.03±0.12 | 1.12±0.14 | 0.455 | 0.55±0.09 | <0.001 | 2.40±0.67 |
Left ventricular volumes and mass are reported as absolute values (mean±standard deviation) and indexed by body surface area. BSA, body surface area; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVIVS, left ventricular interventricular septum; LVM, left ventricular mass; LVPW, left ventricular posterior wall; LVRI, left ventricular remodelling index.
The healthy volunteers and the top‐level athletes were younger than the groups with dilated and hypertrophic cardiomyopathy. The proportions of males and females were the same in all groups. The measured differences concerning LV volumes and wall thickness using 2D echocardiography were significant for each group with the exception of LVEF in groups V and A, left ventricular interventricular septum (LVIVS) in groups V and DC, and LVEDV in groups V and HC.
Feasibility of RT3DE
The acquisition of RT3DE data sets was feasible in all patients except for four patients whose heart size exceeded that of the pyramidal scan volume. These four patients had dilated cardiomyopathy with an extremely large left ventricle. Therefore, these patients were excluded from the analysis, and the data on the remaining 220 patients were examined. The acquisition time was approximately 8 s for each full volume scan, and the time for LV volume and mass quantitative evaluation ranged from 8 to 10 min.
Three‐dimensional echocardiography
Significant differences were found between athletes and healthy volunteers in terms of mean LVEDV (196.2 v 106.4 ml; p<0.001), LVESV (82.8 v 40.8 ml; p<0.001) and LVM (218.8 v 109.1 g; p<0.001). A small but statistically significant difference was also found for the LVEF (57.5 v 61.6%; p = 0.02), while the LVRI was similar in the two groups (1.03 v 1.13; p = 0.455).
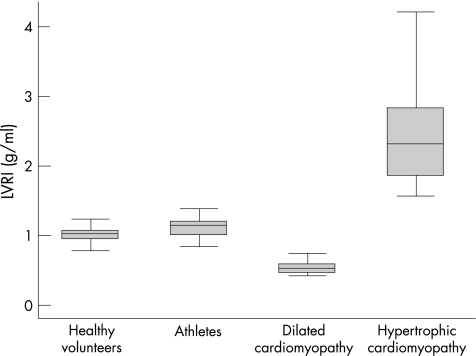
Patients with dilated cardiomyopathy showed significantly higher values for LVEDV (287.1 v 106.4 ml; p<0.001), LVESV (207.8 v 40.8 ml; p<0.001) and LVM (157.5 v 109.1 g; p<0.001) compared with healthy volunteers, while the LVEF values (27.9 v 61.6%; p<0.001) and LVRI (0.55 v 1.03; p<0.001) were significantly lower in the dilated cardiomyopathy group. Finally, patients with hypertrophic cardiomyopathy showed the highest values for LVM (262.1 v 109.1; p<0.001) and LVRI (2.40 v 1.03; p<0.001) while the differences were not significant for LVEDV, LVESV and LVEF compared with the healthy volunteers. Figures 2 and 3 show the distribution of the LVRI in the different groups.
Figure 2 Left ventricular remodelling index (mean values and range) as distributed in the four groups.
Figure 3 Dispersion of the left ventricular remodelling index in the overall population.
Three‐dimensional inter‐ and intra‐observer variability
There was inter‐ and intra‐observer variability. The inter‐observer ICC was 0.60 for LVESV (95% confidence interval (CI) 0.54 to 0.66), 0.63 for LVEDV (95% CI: 0.58 to 0.68), 0.57 for LVEF (95% CI: 0.52 to 0.62) and 0.62 for LVM (95% CI: 0.56 to 0.68).
The intra‐observer ICC was 0.68 for LVESV (95% CI: 0.63 to 0.72), 0.74 for LVEDV (95% CI: 0.70 to 0.80), 0.67 for LVEF (95% CI: 0.62 to 0.72) and 0.72 for LVM (95% CI: 0.68 to 0.76).
Discussion
The novel aspects of this study are: (a) the application of real‐time 3D echocardiography in subjects with varying load conditions and LV function, and (b) the introduction of a new parameter for quantifying left ventricular remodelling, the LVRI.
The LVRI expresses the relationship between LVM and LVEDV. This simple ratio may be extremely useful in differentiating between normal and pathological adaptations of the left ventricle. Moreover, it could have an especially valid application in serial 3D echocardiographic examinations to monitor changes in the left ventricle in patients with cardiovascular disease. The ability to quantify the degree of LV remodelling and monitor its progression has important clinical and research implications in patients with LV volume overload or dysfunction.
In our survey, the healthy volunteers had a balanced ratio between volume and mass that was also found in the athlete population. Thus, despite the high level of conditioning and the development of an increased LVM and LVEDV, the ratio between the two remains constant, thus demonstrating the balanced and appropriate remodelling that occurs in high‐level long‐endurance training.
Dilated cardiomyopathy subjects showed very high values for LVEDV, but LVM did not increase to the same degree. This resulted in significantly lower LVRI values, indicating the eccentric remodelling found in these subjects caused by massive volume overload and progressive chamber dilation not compensated for by an adequate wall‐thickness increase.
Patients with hypertrophic cardiomyopathy showed normal volumes, normal or increased systolic function, and the highest values for LVM. The LVRI was very high in these patients, reflecting the substantial degree of hypertrophy. The highest standard deviation for the LVRI was also found in patients with hypertrophic cardiomyopathy, ranging from 1.56 to 4.21 g/ml, with a mean value of 2.40±0.67 g/ml. This was mostly determined by a high intragroup variability in terms of LVM with a very high range (from 136.8 to 431.6 g).
Left ventricular shape plays an important role in prognostic stratification of patients affected by cardiovascular diseases. Several studies have focused on the adaptation of the left ventricle to pressure or volume overload in various cardiac disorders.26,27,28,29,30 Using echocardiographic methods, the geometry of the left ventricle has been classified on the basis of LVM and relative wall thickness in order to assess changes in its morphology, particularly in patients with systemic hypertension.9,31 The development of increased LVM is widely recognised as a risk factor for cardiovascular events, independently of blood pressure, other cardiac risk factors or the presence of coronary artery disease.32,33,34 M‐mode and 2D techniques have been reported as over‐ and/or under‐estimating LVM, owing to the inadvertent use of oblique cuts or apical foreshortening errors, as well as the use of geometric assumptions.35 Consequently, neither method is sufficiently accurate for measuring small changes in LVM in serial examinations of the same subjects, thereby limiting the clinical usefulness of LVM determination by these techniques. However, these limitations notwithstanding, these techniques are still used in both clinical and research follow‐up of patients for evaluating the effect of drug treatment on LVM regression.
Three‐dimensional echocardiography has been shown to have a high level of accuracy and reproducibility in comparison with cardiac magnetic resonance studies.36,37,38 In a previous study, we verified that real‐time 3D echocardiography is accurate in determining LVM in different load conditions.39 In the current study, we have established that real‐time 3D echocardiographic determination of LVM may be used in abnormal and asymmetric ventricles. This represents a major advantage over 2D echocardiography, which is less accurate and reliable for quantitative analysis of abnormal ventricles.
Information derived from left ventricular diameters, by both M‐mode and 2D echocardiography, has been used to evaluate LV remodelling in patients with valvular or myocardial heart diseases. However, these parameters have failed to predict clinical outcome of patients with and without surgical treatment.40,41,42,43 Other studies have demonstrated that an increase in cardiac size and volume, in the context of impaired LV function, is a negative predictor of long‐term survival after acute myocardial infarction or in heart failure patients.44,45 Recently, Mannaerts et al demonstrated that a sphericity index, derived by real‐time 3D echocardiography, is an earlier and more accurate predictor of remodelling in patients following acute myocardial infarction than other clinical, electrocardiographic or echocardiographic variables.46
In the clinical scenario, there is a need for a reproducible and reliable parameter that expresses modifications in left ventricular geometry for serial assessment of patients over time. In this context, 2D echocardiography, even though widely available for LV assessment, has limited test‐retest variability.47,48 As demonstrated by Jenkins et al, RT3DE is a clinically feasible echocardiographic approach to sequential assessment of LV volume and mass.16 They pointed out that RT3D echocardiography provides low test‐retest variation and high reproducibility of left ventricular measurement between observers. This technique assumes particular importance for use in follow‐up testing.
In the current study, we have demonstrated a good reproducibility of RT3D parameters as regards of inter‐ and intra‐observer variability. The increased accuracy of RT3D echocardiography, in both mass and volume determination, as expressed by the LVRI, would be of value in a large number of clinical applications and in the sequential assessment of changes induced by medical and surgical treatment. Future and further serial echocardiographic examinations of the LVRI trend in different load conditions will be useful in determining the effective role of this proposed index in prognostic stratification of patients affected by various cardiac disorders.
Conclusion
The left ventricular remodelling index, which includes changes in volume and mass, represents a simple and useful parameter for indicating left ventricular adaptations to physiological and pathological conditions.
Abbreviations
A - athletes
3D - three‐dimensional
DC - patients with dilated cardiomyopathy
ECG - electrocardiogram
HC - patients with hypertrophic cardiomyopathy
ICC - inter‐ and intra‐class correlation coefficient
IQR - interquartile range
LVEDV - left ventricular end‐diastolic volume
LVEF - left ventricular ejection fraction
LVESV - left ventricular end‐systolic volume
LVM - left ventricular mass
LVRI - left ventricular remodelling index
RT3DE - real‐time three‐dimensional echocardiography
V - volunteers
95% CI - 95% confidence interval
Footnotes
Competing interests: None.
References
- 1.Cohn J N, Ferrari R, Sharpe N. Cardiac remodelling, concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 200035569–582. [DOI] [PubMed] [Google Scholar]
- 2.Opie L.Heart physiology. Philadelphia, PA: Lippincott Williams and Wilkins, 2004
- 3.Spirito P, Pelliccia A, Proschan M A.et al Morphology of the “athlete's heart” assessed by echocardiography in 947 elite athletes representing 27 sports. Am J Cardiol 199474(8)802–806. [DOI] [PubMed] [Google Scholar]
- 4.Maron B J. Distinguishing hypertrophic cardiomyopathy from athlete's heart: a clinical problem of increasing magnitude and significance. Heart 200591(11)1380–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maron B J, Casey S A, Hurrell D G.et al Relation of left ventricular thickness to age and gender in hypertrophic cardiomyopathy. Am J Cardiol 200391(10)1195–1198. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer M A, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990811161–1172. [DOI] [PubMed] [Google Scholar]
- 7.Hein S, Amon E, Kostin S.et al Progression from compensated hypertrophy to failure in the pressure‐overloaded human heart. Structural deterioration and compensatory mechanisms. Circulation 2003107984–991. [DOI] [PubMed] [Google Scholar]
- 8.Eichhorn P, Grimm J, Koch R.et al Left ventricular relaxation in patients with left ventricular hypertrophy secondary to aortic valve disease. Circulation 1982651395–1404. [DOI] [PubMed] [Google Scholar]
- 9.Ganau A, Devereux R B, Roman M J.et al Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 199219(7)1559–1560. [DOI] [PubMed] [Google Scholar]
- 10.Pouleur H G, Konstam M A, Udelson J E.et al Changes in ventricular volume, wall thickness and wall stress during progression of left ventricular dysfunction. The SOLVD investigators. J Am Coll Cardiol 199322(4A)43A–48A. [DOI] [PubMed] [Google Scholar]
- 11.Doughty R N, Whalley G A, Walsh H A.et al CAPRICORN Echo Substudy Investigators. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy, Circulation 2004109(2)201–206. [DOI] [PubMed] [Google Scholar]
- 12.Van Royen N, Jaffe C C, Krumholz H M.et al Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol 199677843–850. [DOI] [PubMed] [Google Scholar]
- 13.Konstam M A, Kronenberg M W, Rousseau M F.et al Effects of the angiotensin converting enzyme inhibitor enalapril on the long‐term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (Studies of Left Ventricular Dysfunction) Investigators. Circulation 1993882277–2283. [DOI] [PubMed] [Google Scholar]
- 14.Anand I S, Florea V G, Solomon S D.et al Noninvasive assessment of left ventricular remodeling: concepts, techniques, and implications for clinical trials [review]. J Card Fail 20028(6 Suppl)S452–S464. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez‐Chico J L, Zamorano J L, Perez de Isla L.et al Comparison of left ventricular volumes and ejection fractions measured by three‐dimensional echocardiography versus by two‐dimensional echocardiography and cardiac magnetic resonance in patients with various cardiomyopathies. Am J Cardiol 200595(6)809–813. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins C, Bricknell K, Hanekom L.et al Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real‐time three‐dimensional echocardiography. J Am Coll Cardiol 200444(4)878–886. [DOI] [PubMed] [Google Scholar]
- 17.Monaghan M J. Role of real time 3D echocardiography in evaluating the left ventricle. Heart 200692(1)131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhl H P, Schreckenberg M, Rulands D.et al High‐resolution transthoracic real‐time three‐dimensional echocardiography: quantitation of cardiac volumes and function using semi‐automatic border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol 200443(11)2083–2090. [DOI] [PubMed] [Google Scholar]
- 19.Fleming S M, Cumberledge B, Kiesewetter C.et al Usefulness of real‐time three‐dimensional echocardiography for reliable measurement of cardiac output in patients with ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 200595(2)308–310. [DOI] [PubMed] [Google Scholar]
- 20.Maron B J, Douglas P S, Graham T P.et al Task Force 1: preparticipation screening and diagnosis of cardiovascular disease in athletes. J Am Coll Cardiol 200545(8)1322–1326. [DOI] [PubMed] [Google Scholar]
- 21.Maron B J, Ackerman M J, Nishimura R A.et al Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol 2005451340–1345. [DOI] [PubMed] [Google Scholar]
- 22.Corrado D, Basso C, Schiavon M.et al Screening for hypertrophic cardiomyopathy in young athletes. N Eng J Med 1998339364–369. [DOI] [PubMed] [Google Scholar]
- 23.Schiller N B, Shah P M, Crawford M.et al Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐dimensional Echocardiograms. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 24.Klues H G, Schiffers A, Maron B J. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two‐dimensional echocardiography in 600 patients. J Am Coll Cardiol 1995261699–1708. [DOI] [PubMed] [Google Scholar]
- 25.Maron B J, Epstein S E. Hypertrophic cardiomyopathy: a discussion of nomenclature. Am J Cardiol 1979431242–1244. [DOI] [PubMed] [Google Scholar]
- 26.Krumholz H M, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol 199525879–884. [DOI] [PubMed] [Google Scholar]
- 27.Ghali J K, Youlian L, Cooper R S. Influence of left ventricular geometric patterns on prognosis in patients with or without coronary artery disease. J Am Coll Cardiol 1998311635–1640. [DOI] [PubMed] [Google Scholar]
- 28.Bonow R O, Lakatos E, Maron B J.et al Serial long‐term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation 1991841625–1635. [DOI] [PubMed] [Google Scholar]
- 29.Douglas P S, Morrow R, Ioli A.et al Left ventricular shape, afterload, and survival in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 198913311–315. [DOI] [PubMed] [Google Scholar]
- 30.Vasan R S, Larson M G, Benjamin E J.et al Left ventricular dilation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med 19973361350–1355. [DOI] [PubMed] [Google Scholar]
- 31.De Simone G, Di Lorenzo L D, Moccia D.et al Hemodynamic hypertrophied ventricular patterns in systemic hypertension. Am J Cardiol 1987601317–1321. [DOI] [PubMed] [Google Scholar]
- 32.Levy D, Garrison R J, Savage D D.et al Left ventricular mass and incidence of coronary artery disease in an elderly cohort: the Framingham study. Ann Intern Med 1989110101–108. [DOI] [PubMed] [Google Scholar]
- 33.Levy D, Garrison R J, Savage D D.et al Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 19903221561–1566. [DOI] [PubMed] [Google Scholar]
- 34.Ghali J K, Liao Y, Simmons B.et al The prognostic role of left ventricular hypertrophy in patients with and without coronary artery disease. Ann Intern Med 1992117831–836. [DOI] [PubMed] [Google Scholar]
- 35.Myerson S G, Montgomery H E, World M J.et al Left ventricular mass: reliability of M‐mode and 2‐dimensional echocardiographic formulas. Hypertension 200240(5)673–678. [DOI] [PubMed] [Google Scholar]
- 36.Van den Bosch A E, Robbers‐Visser D, Krenning B J.et al Comparison of real‐time three‐dimensional echocardiography to magnetic resonance imaging for assessment of left ventricular mass. Am J Cardiol 200697(1)113–117. [DOI] [PubMed] [Google Scholar]
- 37.Mor‐Avi V, Sugeng L, Weinert L.et al Fast measurement of left ventricular mass with real‐time three‐dimensional echocardiography: comparison with magnetic resonance imaging. Circulation 2004110(13)1814–1818. [DOI] [PubMed] [Google Scholar]
- 38.Oe H, Hozumi T, Arai K.et al Comparison of accurate measurement of left ventricular mass in patients with hypertrophied hearts by real‐time three‐dimensional echocardiography versus magnetic resonance imaging. Am J Cardiol 200595(10)1263–1267. [DOI] [PubMed] [Google Scholar]
- 39.De Castro S, Pelliccia A, Caselli S.et al Remodeling of left ventricle in athlete's heart: a three dimensional echocardiographic and magnetic resonance imaging study. Heart 200692975–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enriquez‐Sarano M, Tajik A J, Schaff H V.et al Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation 199490830–837. [DOI] [PubMed] [Google Scholar]
- 41.Ramanathan K B, Knowles J, Connor M J.et al Natural history of chronic mitral insufficiency: relation of peak systolic pressure/end‐systolic volume ratio to morbidity and mortality. J Am Coll Cardiol 198431412–1416. [DOI] [PubMed] [Google Scholar]
- 42.Ling L H, Enriquez‐Sarano M, Seward J B.et al Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 19963351417–1423. [DOI] [PubMed] [Google Scholar]
- 43.Bonow R O, Rosing D R, McIntosh C L.et al The natural history of asymptomatic patients with aortic regurgitation and normal left ventricular function. Circulation 198368509–517. [DOI] [PubMed] [Google Scholar]
- 44.White H D, Norris R M, Brown M A.et al Left ventricular end‐systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 19877644–51. [DOI] [PubMed] [Google Scholar]
- 45.St John Sutton M, Pfeffer M A, Plappert T.et al Quantitative two‐dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation 199489(1)68–75. [DOI] [PubMed] [Google Scholar]
- 46.Mannaerts H F, van der Heide J A, Kamp O.et al Early identification of left ventricular remodelling after myocardial infarction, assessed by transthoracic 3D echocardiography. Eur Heart J 200425680–687. [DOI] [PubMed] [Google Scholar]
- 47.Bottini P B, Carr A A, Prisant L M.et al Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 19958221–228. [DOI] [PubMed] [Google Scholar]
- 48.Gopal A S, Shen Z, Sapin P M.et al Assessment of cardiac function by three‐dimensional echocardiography compared with conventional noninvasive methods. Circulation 199592842–853. [DOI] [PubMed] [Google Scholar]