Management of tricuspid regurgitation (TR) is becoming an increasingly difficult decision‐making problem. TR occurs in 8–35% of patients, especially in association with acquired left heart valve disease of rheumatic origin, primary isolated TR being very rare. It is more frequently found in association with mitral rather than with aortic valve disease, and is much rarer in degenerative disease. In the majority of patients (70–85%), the TR is said to be “functional”, caused by dilatation of the annulus as a result of increased pulmonary and right ventricular pressure; in the remaining 15–30% of the cases it may be organic and related to direct involvement of the tricuspid valve by the rheumatic disease.1,2 Whichever type, TR has a significant impact on the clinical condition and the medium and long‐term prognosis of the patients. Hence, it requires special consideration during mitral and/or aortic valve surgery and thereafter.
In 1967, Brawnwald et al3 advised a conservative (no touch) approach to TR. Indeed, it was thought that appropriate correction of the left‐sided valve disease would most probably result in a decrease or even abolition of the so‐called functional TR. However, experience has shown that TR does not always disappear, and may increase, especially when the mitral and/or aortic valve disease is not completely or adequately resolved during surgery, as happens sometimes in cases with less than perfect correction of mitral regurgitation or stenosis. Furthermore, isolated severe TR is now increasingly observed in patients with normal left heart valve function after either mitral valve annuloplasty or replacement. The true incidence of secondary TR is not well known, but it has led some to question the functional nature of TR, accompanying left‐sided valve disease.
Even moderate TR observed during surgery of left heart valves may not regress spontaneously, especially when there is already a degree of right ventricular dysfunction indicated by annular dilatation. In their experience with patients who were subjected to valvuloplasty for mitral valve regurgitation, Dreyfus et al4 found that the tricuspid annulus was abnormally dilated in about 50% of the patients, even in the absence of TR.
PHYSIOPATHOLOGY
For more than 25 years, we have been aware of tricuspid annular dilatation and indeed significant TR in a large number of rheumatic patients subjected to mitral valve surgery.5,6,7 We have shown that the initial and principal pathological process affecting the mitral valve in acute rheumatic carditis is dilatation of the annulus. In other words, the mitral annulus is abnormally weak due to rheumatic involvement.6,8,9,10,11 It is therefore not surprising that the tricuspid annulus is similarly affected in some instances.
Others have demonstrated that loss of contraction of the myocardium surrounding the annulus is the leading mechanism of TR.12 We learnt, in fact, that so‐called “functional” TR occurs in rheumatic heart disease only where there is severe pulmonary hypertension and the amount of regurgitation is relatively small.3,10 “Functional” is a misleading term when used in the context of tricuspid valve disease. As with the mitral valve, annular pathology plays as important a role in causing valve malfunction as do leaflet, chordal or papillary muscle disease. Haemodynamically significant TR before rheumatic mitral valve surgery should not be regarded as functional, nor should it be anticipated that the TR will resolve after successful mitral surgery. Immediately after such surgery, the TR may appear relatively mild and easily controlled with diuretics. After months or years, however, forceful systolic waves and a pulsatile liver supervene.6,13
TR is often a progressive condition. With the increased volume of TR, the right ventricle, as well as of course the right atrium, dilate. Eventually the right ventricular systolic function “fails”, the diastolic pressure rises and the interventricular septum moves towards the left ventricle during diastole. This is an important example of cardiac chamber inter‐relationships in which elevated pressure and expanding volume of one chamber compress, or constrain, the adjacent chamber and hence raises the pressure in the latter. These interrelated ventricular cavity pressure and volume alterations are essential features of the “restriction‐dilatation syndrome”.6,13,14 We named it restriction‐dilatation syndrome because decreased left ventricular cavity size, aggravated sometimes by fibrous adhesions following previous surgery, especially left thoracotomy, has raised left ventricular diastolic pressures and results in a rise in pulmonary venous pressure. Naturally, this perpetuates and increases the TR. It is the essential feature predisposing to and later aggravating the restriction‐dilatation syndrome. Tricuspid valve surgery carries a high surgical mortality when performed in the context of the restriction‐dilatation syndrome with severe TR. An aggressive approach to the tricuspid valve at the time of rheumatic mitral valve surgery has, for practical purposes, prevented this previously prevalent postoperative complication in our institutions.
Right ventricular dysfunction and TR are more common in mitral than in aortic valve disease. Lowering of pulmonary vascular resistance, generally increased in mitral valve disease, is essential for reversal of TR. A longer clinical course could result in a greater degree of clinical and haemodynamic deterioration, thus with increased surgical risk. In the presence of right ventricular failure, operative mortality increases from 5% to 11% and from 8% to 22% during follow‐up.15 On the other hand, improvement of symptoms is less common in valvular patients with preoperative right ventricular dysfunction (ejection fraction <30%).16 Finally, persisting TR following mitral valve replacement is associated with an impaired cardiac output response to exercise.17 Hence, recent evidence demonstrates that functional TR can be ignored only in patients with predictable and significant reduction in the pulmonary resistance, which usually follows earlier correction of left‐side pathology.
INDICATIONS FOR SURGERY OF THE TRICUSPID VALVE
When should one repair/replace the tricuspid valve in association with surgery of left‐sided heart valve disease?
There are a certain number of problems that complicate the decision. Firstly, there is no reliable method to judge how much of the TR is reversible when the left heart valve dysfunction is corrected. Secondly, there is a lack of reliable and repeatable methods of measuring and quantifying the degree of TR; it is certainly more difficult than with the mitral valve and one cannot rely on echocardiography alone. The clinical assessment is extremely important here. Finally, there is no satisfactory method to assess true right ventricular function.
Some other questions remain unanswered. What is the choice between repair and replacement? What is the effectiveness of different methods of repair? What type of prosthesis is to be used if the valve is replaced? What can be done with mild to moderate tricuspid incompetence? Finally, how do you predict those patients who will return after mitral valve surgery with persistent, sometimes very bothersome, TR?
In their recent paper, Dreyfus et al4 dealt with the problem of secondary tricuspid dilatation, with or without regurgitation (should it be corrected?). These authors reviewed their experience with patients who were subjected to valvuloplasty for mitral valve regurgitation and observed echocardiographically, in about 50% of their total series, that the tricuspid annulus was abnormally dilated although TR was seldom demonstrated. Two groups of patients were compared retrospectively: in one group, patients had a simultaneous tricuspid annuloplasty, while in the other group the tricuspid valve was left undisturbed. The authors found that in the late follow‐up, the patients who had tricuspid annuloplasty had a progressive decrease in the degree of regurgitation, while patients who did not have a tricuspid annuloplasty had progressive tricuspid dysfunction.
Factors in the genesis of the restrictive‐dilatation syndrome
Mitral valve dysfunction
Myocardial dysfunction or other reason for left ventricular dilatation
Pulmonary thromboembolic disease and chronic obstructive airways disease
Functional or organic tricuspid regurgitation
Pericardial restriction or constriction
Consequently, they proposed that tricuspid annuloplasty be done at the time of mitral valve surgery when the diameter of the annulus is greater than 21 mm/m2, and this was found to improve functional capacity without increasing perioperative morbidity and/or mortality. Basically, what these authors are proposing is “prophylactic” annuloplasty in order to avoid progression of the disease. Their numbers are convincing but require validation by other authors' experiences. But in recent years we have been increasingly aggressive to the tricuspid valve. Annuloplasty adds only a very short time to cardiopulmonary bypass and aortic crossclamping (it can even be done after release of the aortic crossclamp) and, at least in our experience, does not seem to add significantly to perioperative mortality and morbidity (see below).
This problem is especially pertinent in patients with mitral stenosis. Wong et al18 and other authors have also dealt with this subject and recommended that annuloplasty be performed at least in TR grades II and III. Also from the international guidelines, there is some evidence that annuloplasty should be performed for more than mild TR, especially in patients with pulmonary hypertension secondary to mitral valve disease requiring surgery.
LATE OCCURRING TRICUSPID REGURGITATION
Late appearance of TR requiring reoperation is exemplified by a patient who has been subjected to mitral surgery and presents weeks, months or years later with symptoms and signs of right and/or left heart failure, and significant TR. In the past 20 years, we have had to proceed to reoperation for isolated tricuspid valve annuloplasty in many patients previously subjected to surgery of left heart valve disease. This procedure is associated with a significant surgical risk.
What are the risk factors for persisting or worsening TR after a mitral valve procedure without surgery to the tricuspid valve? First is the mechanism, discussed above, of persisting or recurrent mitral valve disease which predisposes to tricuspid dysfunction. On the other hand, the more severe the TR already present at the time of the first surgery, the more likely it is to persist or to increase late after mitral valve surgery. Mild TR rarely persists or progresses, whereas moderate or severe TR may and usually does so. Finally, longstanding, perhaps irreversible, right ventricular dilatation secondary to mitral valve or pulmonary vascular disease probably also predisposes to persistent TR. All these factors take part in the restriction‐dilatation syndrome described above.
Therefore, TR that does not disappear immediately after surgery with intensive pre‐ and postoperative anti‐congestive therapy is more likely to persist postoperatively if not repaired. On the other hand, organic TR, usually associated with some degree of stenosis, is more likely to persist than is “functional” TR.
Late TR appears to be preferentially associated with rheumatic heart disease. In a recent study of 264 patients who had mitral valve repair at Papworth Hospital, Cambridge, where just over 80% had had degenerative mitral regurgitation, significant TR was not detected clinically 1–11 years after mitral valve repair.19 It must be recognised, however, that specific clinical observations on the tricuspid valve were not a principal goal of the study and that diuretic treatment may have masked moderate TR in some instances. On the other hand, Matsunaga and Duran20 found that functional TR is frequently associated with functional ischaemic mitral regurgitation. In their experience, close to 50% of patients have TR after mitral valve repair and the incidence of postoperative TR increased with time. They hypothesised that preoperative tricuspid annulus dilation might be a predictor of late TR.
It certainly would be contributory to thousands of patients with rheumatic heart disease if surgeons and cardiologists would refute the prolonged and ongoing dogma that “functional” TR will subside after appropriate left‐sided rheumatic valve surgery and that they practise an aggressive policy towards performing, even prophylactically, tricuspid annuloplasty at the time of such surgery. Nonetheless, based on currently available data, an indication for tricuspid annuloplasty at the time of mitral surgery for degenerative disease can barely be substantiated.
So, what are the surgical considerations when dealing with the problem of late tricuspid valve regurgitation?
In these cases, there are two situations to consider: is this a first tricuspid valve procedure—that is, originally the patient had only mitral or aortic valve surgery—or was there an intervention on the tricuspid valve already during the first procedure, in which case it is a repeat tricuspid valve operation? In either case, the patient may also present with another valve disease, or otherwise it may be an isolated problem of TR, a problem which we see with increasing frequency.
It is quite clear that redo heart surgery of any sort is associated with a higher risk, which is magnified by the number of times it takes place. Hospital mortality for repeat tricuspid valve surgery in patients who had a prior cardiac operation may reach 50%, although it is usually described between 10–20%.21 Surgery in patients with isolated organic TR should, therefore, be delayed unless there is a good reason to believe that a successful valvuloplasty can be performed or the patient continues to show signs of cardiac failure despite adequate diuretic treatment. Aggressive anti‐failure therapy is indicated and should be maximised to the limits of patient tolerance. We further believe that long‐term, perhaps indefinite, anti‐failure therapy may avoid or at least retard the development of significant TR.
High functional class, severe right heart failure, low right ventricular ejection fraction, high pulmonary pressure and pulmonary arterial resistance are additional risk factors when repeating tricuspid surgery. As mentioned, large right ventricular volumes specifically affect the risk for re‐entry sternotomy, but this may be obviated by adequate surgical technique, as discussed below.
FOLLOW‐UP AND DIAGNOSIS
Patients who have undergone mitral valve surgery are routinely subjected to close follow‐up. Patients who have had tricuspid valve surgery or have TR which was not completely resolved after left‐sided valve surgery require no special considerations, but should be followed more closely.
Clinical detection of TR is simple and accurate.6,13 Raised jugular venous pressure, hepatomegaly, pulsatile liver and hepato‐jugular reflux are followed by ankle swelling and, later, by ascites and generalised oedema. The characteristic systolic wave is both visible and palpable in the neck veins. On auscultation, a systolic murmur, variable with respiration, is usually present. Echocardiographic examination confirms the diagnosis and is useful to assess the size of the right cardiac chambers and right ventricular function. In severe TR there is usually dilatation of both the right atrium and ventricle. Retrograde flow in the vena cava is synonymous with severe TR.
TR is probably partially related to the enlargement of the right‐sided heart cavities and to alterations in the geometry of the right ventricle, and a decrease in the volaemia may help to obviate the problem. We must emphasise that TR is not benign. It is often self‐perpetuating, progressive and becomes very incapacitating to the patient who has to receive massive doses of diuretics or be subjected to high‐risk surgery.
Once the patient becomes symptomatic, aggressive anti‐failure therapy with furosemide is indicated—usual doses are 40–160 mg daily. Spironolactone appears especially indicated in patients with TR and in right ventricular failure. Dosages of 25–100 mg daily may be utilised, depending on the clinical situation and on the response. We are well aware that diuretic treatment may reduce the haemodynamic severity of TR by relieving right ventricular distension and hence lowering right atrial pressure. If this is not sufficient, consideration must be given to isolated surgery of the tricuspid valve.
SURGICAL TECHNIQUE
In the tricuspid position, prosthetic valve substitutes have at least the same type and degree of complications as in the aortic and the mitral positions. Mechanical prostheses have an increased incidence of thrombo‐embolic phenomena, while bioprostheses have the problem of early and late degeneration, depending on the age of the patient. The use of mechanical prostheses in the tricuspid position in multi‐valvular procedures has been associated not only with increased mortality but also with a high incidence of thrombotic complications, valve dysfunction from tissue ingrowth and, in the case of cage and ball valves, incorporation of the cage into the wall of the right ventricle. Although thrombolytic treatment has, in recent years, been successfully applied in the treatment of thrombosis of tricuspid mechanical prostheses, it remains a very serious and often lethal problem. Survival curves show that by five years only about 35–45% of the patients were alive free from reoperation after tricuspid valve replacement.22
On the other hand, partial or total replacement of the tricuspid valve with homografts has been suggested and has been done quite extensively by some groups, but the results are still not homogeneous and, in some cases, controversial.
But it appears that only exceptionally does the tricuspid valve need to be replaced as a first procedure, because the valve tolerates well a less than perfect repair, in contrast to what happens with the aortic and the mitral valves where total competence is of primordial importance. Hence, in the minds of almost everybody, annuloplasty is the surgery of choice when dealing with most forms of TR.
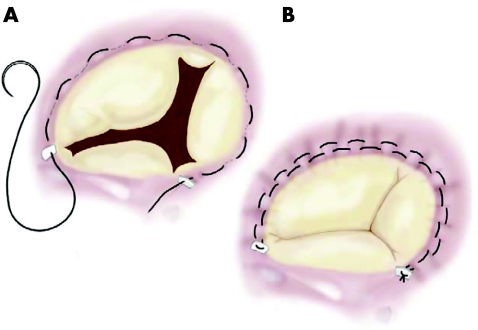
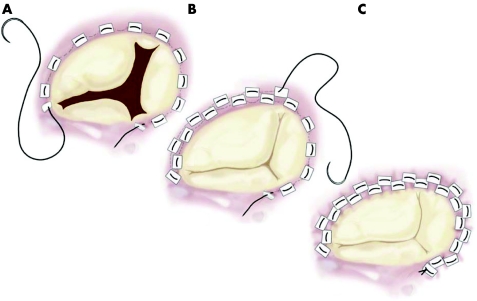
In the early 1970s, Deloche et al,23 from Carpentier's group, clearly demonstrated that dilatation of the tricuspid annulus occurs almost entirely in the mural portion of the annulus; based also on his own findings, DeVega24 developed his very well known procedure, which consists of plication of the posterior and anterior portion of the annulus, preserving the septal portion, with a double continuous suture (fig 1). This procedure has since been used in tens of thousands of cases by most surgeons throughout the world and it appears to be safe and efficacious. Sometimes, however, the sutures may pull out of the tissues and the guitar‐string syndrome occurs. In order to avoid this type of complication, in 1987 Antunes and Girdwood25 described a modification of the procedure which consisted of the interposition of Teflon pledgets in each bite of the suture, but the concept remains exactly the same (fig 2).26 Other methods of suture‐annuloplasty have been described and used by some groups, but the DeVega annuloplasty or its modifications has gained wider acceptance. In these situations, the valve is usually made mildly stenotic, but it has been emphasised that tricuspid stenosis is a relatively benign lesion, usually well tolerated by the patient.6,13
Figure 1 Classical DeVega annuloplasty.
Figure 2 Authors' modification of the DeVega annuloplasty.
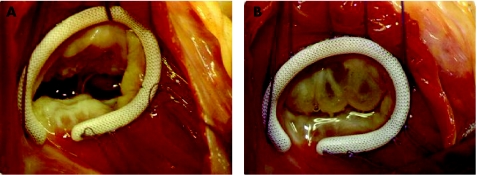
The routine use of an annuloplasty ring, whether pre‐shaped, rigid or semi‐rigid, as the Carpentier ring, or flexible, as the Duran ring, has been considered superior and recommended by many groups.27 However, in our experience, the implantation of a ring is specifically indicated when there is organic involvement of the tricuspid valve, usually with stenosis, where commissurotomy is also necessary. In these circumstances, because remodelling of the annulus is probably essential, we prefer the Carpentier ring (fig 3). In all other cases, we use the modified DeVega annuloplasty.28
Figure 3 Intraoperative photograph after annuloplasty with a Carpentier‐ Edwards ring.
Somewhat different is the problem of late appearing TR. In most repeat operations, morbidity and even mortality is often related to the re‐entry. Classical median sternotomy may be used, but a right thoracotomy may be advantageous for a second and third reoperation. On the other hand, the adhesions of the pericardium, together with extreme dilatation of the right atrium and ventricle, may dictate the need for using peripheral artery (for example, femoral) and even vein cannulation in some cases, especially in multiple reoperations, although we have not had to utilise it frequently. We still use the median sternotomy and the classical vena cavae–aortic cardiopulmonary bypass in the vast majority of cases.
If it had not been done previously, annuloplasty is, again, preferable to valve replacement. In these cases, however, greater tendency for deformity of the whole valve mechanism, rather than isolated annular dilatation, may dictate more extended use of a prosthetic ring.
SURGICAL RESULTS
In our experience, the modified DeVega tricuspid annuloplasty proved to be a safe and efficacious procedure for the management of secondary TR. It is technically easy and reproducible, even by relatively inexperienced surgeons. In our view, it should be used in all patients with more than mild “functional” TR. We26,28 have recommended and followed this policy for more than a decade with encouraging results, inasmuch as we have observed a low rate of late reoperations.
Beginning March 1988 through December 2004, 495 patients were subjected to surgery of the tricuspid valve, together with surgery of the mitral valve, in our department in Coimbra, Portugal. Only five patients required valve replacement. Of the remainder, 48 (9.7%) had implantation of a Carpentier ring and 442 had the modified DeVega procedure. The 30‐day mortality for patients who had tricuspid annuloplasty was 1.6% as compared with 1.0% in those without tricuspid valve surgery. To our knowledge, only three patients required reoperation for persistent or recurrence of severe TR and no case of dehiscence of the annuloplasty suture was identified.
Management of tricuspid valve regurgitation: key points
Tricuspid regurgitation (TR) is common in patients with left‐sided heart (especially mitral) valve disease
There is now some evidence that, in these cases, TR is not always “functional”; it may be due to pathological involvement of the annulus
Therefore, correction of left‐sided heart valve disease does not automatically correct TR
Dilatation of the tricuspid annulus is progressive and may not be accompanied by TR initially, but eventually leads to it; “prophylactic intervention may be justified
Tricuspid annuloplasty achieves excellent results; tricuspid valve replacement is seldom necessary
Suture annuloplasty is usually adequate but ring annuloplasty is essential in organic TR
We believe that the modified technique, almost abolishing suture dehiscence, may have contributed significantly toward the difference between our experience and that recently reported by McCarthy and co‐authors.27
On the other hand, reoperation for late TR, whether isolated or in conjunction with repeat surgery of another valve, has an operative risk which is higher than that which occurs after other redo valve surgery and may reach 10–20%, although much lower mortality rates have recently been reported.29,30 Preoperative preparation to reduce cardiac failure, and physiotherapy, adequate anaesthesia and surgical technique may contribute to reduce the risk. Special attention must be given to renal function, as significant renal failure dramatically increases the surgical risk. Because surgery is rarely required on an emergency basis, all measures that may improve the patient's condition before surgery are justified.
CONCLUSION
It is obvious that functional TR can be ignored only in patients with a predictable and significant reduction in pulmonary resistance. The quality of the repair or replacement of the left‐sided valvulopathy appears fundamental to avoid late TR. Secondary dilatation of the tricuspid annulus without regurgitation is present in a significant number of patients with severe mitral regurgitation and is a progressive disease which often does not resolve with correction of the primary lesion alone. Tricuspid annuloplasty at the time of mitral valve surgery improves functional capacity without significantly increasing perioperative morbidity and mortality.
It is, thus, appropriate to recommend that surgeons be more liberal in the indications for tricuspid annuloplasty. It should be performed if the tricuspid insufficiency is more than mild and one or more of the following factors are present: the valve is rheumatic, the size of the annulus is >21 mm/m2, there is dilatation of the right chambers and inferior vena cava, and there is right ventricular overload. If TR occurs or progresses late after valve surgery, aggressive medical treatment is indicated but surgery of the tricuspid valve may be required. Although it carries a higher operative risk, patients usually show significant clinical improvement.
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
References
- 1.Simon R, Oelert H, Borst H G.et al Influence of mitral valve surgery on tricuspid incompetence concomitant with mitral valve disease. Circulation 198062(suppl l)S152–S157. [PubMed] [Google Scholar]
- 2.King R M, Schaff H V, Danielson G K.et al Surgery for TR late after mitral valve replacement. Circulation 198470(suppl l)S193–S197.This is one of the first important series of patients reoperated on for secondary tricuspid regurgitation late after mitral valve surgery, drawing attention to the importance and prevalence of this complication. [PubMed] [Google Scholar]
- 3.Braunwald N S, Ross J, Jr, Morrow A G. Conservative management of TR in patients undergoing mitral valve replacement. Circulation 196735(suppl I)63–69. [DOI] [PubMed] [Google Scholar]
- 4.Dreyfus G, Corbi P J, John C K M.et al Secondary TR or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 200579127–132.The authors found that in about 50% of the cases of mitral valve repair the tricuspid annulus was abnormally dilated, even in the absence of TR. In the late follow‐up, the patients who did not have a tricuspid annuloplasty had progressive tricuspid dysfunction. They recommend “prophylactic” tricuspid annuloplasty. [DOI] [PubMed] [Google Scholar]
- 5.Barlow J B, Pocock W A, Meyer T E. Conditions relevant to assessment or function of the mitral valve. 2. Tricuspid valve disease. In Barlow JB, ed. Perspectives on the mitral valve Philadelphia, FA Davis Co, 1987;338–59
- 6.Barlow J B. Aspects of mitral and tricuspid regurgitation. J Cardiol 1991213–33. [PubMed] [Google Scholar]
- 7.Barlow J B. Mitral valve disease: a cardiologic‐surgical interaction. Isr J Med Sci 199632831–832. [PubMed] [Google Scholar]
- 8.Barlow J B, Kinsley R H, Pocock W A. Rheumatic fever and rheumatic heart disease. In: Barlow JB, ed. Perspectives on the mitral valve. Philadelphia: FA Davis Co, 1987;227–45
- 9.Barlow J B, Marcus R H, Pocock W A.et al Mechanisms and management of heart failure in active rheumatic carditis. S Afr Med J 199078181–186. [PubMed] [Google Scholar]
- 10.Barlow J B. Aspects of active rheumatic carditis. Aust NZ J Med 199222592–600. [DOI] [PubMed] [Google Scholar]
- 11.Marcus R H, Sareli P, Pocock W A.et al The spectrum of severe rheumatic mitral valve disease in a developing country. Correlations among clinical presentation, surgical pathological findings and hemodynamic sequelae. Ann Intern Med 1994120177–183. [DOI] [PubMed] [Google Scholar]
- 12.Simon R. Size and motion of the tricuspid annulus. Circulation 198367709. [DOI] [PubMed] [Google Scholar]
- 13.Barlow J B. Aspects of tricuspid valve disease, heart failure and the “restriction‐dilatation syndrome”. Rev Port Cardiol 199514991–1004. [PubMed] [Google Scholar]
- 14.Pocock W A, Antunes M J, Sareli P.et al Surgical aspects of mitral valve disease. 2. Late postoperative course and complications: emphasis on the “restriction‐dilatation syndrome”, In: Barlow JB, ed. Perspectives on the mitral valve. Philadelphia: FA Davis Co 1987270–288.This landmark chapter describes the “restriction‐dilatation syndrome” which is characterised by interrelated ventricular cavity pressure and volume alterations. With progressive TR, the right ventricle and the right atrium dilate. Eventually, the right ventricular systolic function “fails”, the diastolic pressure rises and the interventricular septum moves towards the left ventricle during diastole.
- 15.Pinzani A, de Gevigney G, Pinzani V.et al Pre‐ and postoperative right cardiac insufficiency in patients with mitral or mitral‐aortic valve diseases. Arch Mal Coeur Vaiss 19938627–34. [PubMed] [Google Scholar]
- 16.Borer J S, Hochreiter C, Rosen S. Right ventricular function in severe non‐ischaemic mitral insufficiency. Eur Heart J 199112(suppl B)22–25. [DOI] [PubMed] [Google Scholar]
- 17.Groves P H, Hall R J C. Late TR following mitral valve surgery. J Heart Valve Disease 1992180–86.In this paper, the authors show evidence that persisting TR following mitral valve replacement is associated with impaired cardiac output response to exercise which worsens as the tricuspid dysfunction increases, eventually requiring reoperation. [PubMed] [Google Scholar]
- 18.Wong M, Matsumura M, Kutsuzawa S.et al The value of Doppler echocardiography in the treatment of TR in patients with mitral valve replacement. Perioperative and two‐year postoperative findings. J Thorac Cardiovasc Surg 1990991003–1010. [PubMed] [Google Scholar]
- 19.Lim E, Ali Z A, Barlow C W.et al Determinants and assessment of regurgitation after mitral valve repair. J Thorac Cardiovasc Surg 2002124911–917.This is a follow‐up study of 264 patients who had mitral valve repair for degenerative mitral regurgitation. Significant TR was not detected clinically up to 11 years after the mitral valve surgery, indirectly proving that this complication occurs almost exclusively in rheumatic cases. [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga A, Duran C M. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation 2005112(9 suppl)I453–I457. [DOI] [PubMed] [Google Scholar]
- 21.Hornick P, Harris P A, Taylor K M. Tricuspid valve replacement subsequent to previous open heart surgery. J Heart Valve Dis 1996520–25. [PubMed] [Google Scholar]
- 22.Reed G E, Boyd A D, Spencer F C.et al Operative management of tricuspid regurgitation. Circulation 197654(suppl 3)96–98. [PubMed] [Google Scholar]
- 23.Deloche A, Guerinon J, Fabiani J N.et al Anatomical study of rheumatic tricuspid valve diseases: application to the study of various valvuloplasties. Ann Chir Thorac Cardiovasc 197312343–349.In this classic paper, the authors demonstrated that dilatation occurs mostly in the mural part of the tricuspid annulus, the septal portion remaining practically unchanged. This formed the basis for most techniques of tricuspid annuloplasty. [PubMed] [Google Scholar]
- 24.DeVega N G. La anuloplastia selective, reguable y permanente. Rev Esp Cardiol 1972256–9.In this historical paper, DeVega makes the first description of the most used technique of tricuspid annuloplasty. [PubMed] [Google Scholar]
- 25.Antunes M J, Girdwood R W. Segmental tricuspid annuloplasty: a modified technique. Ann Thorac Surg 198335676–678. [DOI] [PubMed] [Google Scholar]
- 26.Antunes M J. Segmental tricuspid annuloplasty revisited (letter). J Thorac Cardiovasc Surg 19921031025. [PubMed] [Google Scholar]
- 27.McCarthy P M, Bhudia S K, Rajeswaran J.et al Tricuspid valve repair: durability and risk factors for failure (see also discussion). J Thorac Cardiovasc Surg 2004127674–685.In this paper, originating from the Cleveland Clinic, the authors demonstrated that in their experience ring annuloplasty of the regurgitating tricuspid valve is superior to non‐ring methods, but this has not been generally accepted (discussion). [DOI] [PubMed] [Google Scholar]
- 28.Antunes M J. DeVega annuloplasty of the tricuspid valve. Operative Techniques in Thoracic and Cardiovascular Surgery 20038169–176. [Google Scholar]
- 29.Xiao X, Huang H, Zhang J.et al Surgical treatment of late tricuspid regurgitation after left cardiac valve replacement. Heart Lung Circ 20041365–69. [DOI] [PubMed] [Google Scholar]
- 30.Izumi C, Iga K J, Konishi T. Progression of isolated tricuspid regurgitation late after mitral valve surgery for rheumatic mitral valve disease. J Heart Valve Dis 200211353–356. [PubMed] [Google Scholar]