Abstract
Chronic obstructive pulmonary disease (COPD) is a progressive condition characterized by airflow obstruction which ultimately kills many patients. It is common in both men and women and there is a 24-30% 5-year survival rate in the UK for those with severe disease. The annual death rate in the UK from COPD approaches that from lung cancer. Patients' symptoms can be improved by drug therapy, but stopping smoking is also an effective way of improving the outcome in patients at all stages of COPD.
Predicting prognosis has been difficult in COPD due to the variable illness trajectory. However, assessment of severity of lung function impairment, frequency of exacerbations and requirement for long term oxygen therapy can help identify patients entering the final 12 months of life. Symptom burden and impact on activities of daily living for patients with COPD are comparable with that of cancer patients, and palliative care approaches are equally necessary, yet few publications exist to guide clinicians in this area. An evidence base exists for the management of dyspnoea with oxygen therapy and opioid drugs. There is less evidence for the effective treatment of depression and anxiety, fatigue and pain, and treatment is based on experience and considered best practice. This review discusses the problems that patients experience and offers practical guidance.
The management of patients should be shared between primary and secondary care, with multidisciplinary teams being involved at an early stage. Patients and their families require honest and clear communication about the condition and what to expect in the future. The strict application of advance care planning and directives may not be feasible or appropriate, but there is evidence that attitudes towards resuscitation and artificial ventilation can be explored without distress. The requirement by patients and carers for surveillance and timely support is acknowledged, but how to provide such input is as yet unclear, with little evidence to support the widespread implementation of nurse-led management interventions. The hospice movement has become increasingly involved in the management of life-threatening, non-malignant disease and should be involved in the multidisciplinary care of patients dying from COPD.
INTRODUCTION
This review is aimed at clinicians and health care professionals providing end-of-life care in the community for patients suffering from severe, end-stage chronic obstructive pulmonary disease (COPD). This patient group spend the majority of the final year of life in the community and have frequent contact with the primary health care team. Guidelines for active and supportive treatment for patients with COPD have been published1 and are not the focus of this review. There is often no clearly identifiable point at which management changes from active supportive therapy to palliative care, and it is usually a matter of experienced clinical judgement. Some therapies, such as bronchodilator therapy, remain as important in palliative care, others such as oxygen therapy may take on a greater role for symptom control, while still others, such a pulmonary rehabilitation, are less likely to be relevant as part of palliative care.
COPD is a common chronic disease which causes significant mortality and morbidity. The characteristic symptoms are worsening breathlessness and exercise limitation, and progressive deterioration of health status eventually leading to death.
Comprehensive definitions have been produced by the National Institute of Clinical Excellence (NICE) in the UK (Box 1),1 as well as by the Global Initiative for Chronic Obstructive Lung Disease (GOLD)2 and by the American Thoracic Society and European Respiratory Society in their joint guidelines.3
There is no single diagnostic test for COPD. The diagnosis relies on clinical judgment based on a combination of history, physical examination and confirmation of air flow obstruction using spirometry. In COPD there is an irreversible decline of forced expiratory volume in one second (FEV1).1
The best available data suggest there are approximately 700 000 patients diagnosed with COPD in England and Wales.4 Allowing for known levels of under-diagnosis, the true number of patients with COPD is likely to be around 1.5 million.1 Most patients are not diagnosed until they are in their fifties and the prevalence increases with increasing age.
Box 1 National Institute for Clinical Excellence (NICE) definitions1
Chronic obstructive pulmonary disease (COPD) is characterized by airflow obstruction. The airflow obstruction is usually progressive, not fully reversible and does not change markedly over several months. The disease is predominantly caused by smoking.
Airflow obstruction is defined as a reduced FEV1 (forced expiratory volume in 1 second) and a reduced FEV1/FVC ratio (where FVC is forced vital capacity), such that FEV1 is less than 80% predicted and FEV1/FVC is less than 0.7. The airflow obstruction is due to a combination of airway and parenchymal damage.
The damage is the result of chronic inflammation that differs from that seen in asthma and which is usually the result of tobacco smoke.
Significant airflow obstruction may be present before the individual is aware of it.
COPD produces symptoms, disability and impaired quality of life which may respond to pharmacological and other therapies that have limited or no impact on the airflow obstruction.
COPD is now the preferred term for patients with airflow obstruction who were previously diagnosed as having chronic bronchitis or emphysema.
Other factors, particularly occupational exposures, may also contribute to the development of COPD.
The five-year survival from diagnosis in the UK is 78% in men and 72% in women with mild disease, but falls to 30% in men and 24% in women with severe disease.5 The mean age of death of UK patients with severe COPD is 74.2 years, compared with 77.2 years in patients with mild disease and 78.3 years in non-COPD controls (Soriano, personal communication).
It is difficult to be certain of the true mortality due to COPD. Some patients die with the disease rather than because of it and others will die of causes related to COPD, but their death may be certified as being due to these complications.6 Analysis of trends in death rates is also complicated by changes in the diagnostic labels. National Statistics suggest that there were 27 932 deaths due to COPD in the UK in 1999.7 This represents 5.1% of all deaths (4.3% of all male deaths and 5.9% of all female deaths). In men, age standardized mortality rates from COPD have fallen progressively over the last 30 years, but in women there has been a small but progressive increase over the last 20 years.8
With an ageing population, many of whom will have smoked for a considerable part of their lives and are therefore at high risk of developing COPD, the application of palliative care to COPD will be very important.
NATURAL HISTORY
The loss of lung function in smokers susceptible to COPD may remain asymptomatic for many years, despite significant structural damage and functional change.9 Functional impairment and disability often appear to develop fairly rapidly in patients in their late forties and fifties when the pulmonary reserve has been exhausted. The relationship between loss of lung function and the development of breathlessness varies greatly from individual to individual10 and is influenced by the extent to which patients avoid activities that they know will provoke breathlessness. This makes it particularly difficult to classify the severity of COPD clinically,11 but it is usually possible for experienced clinicians to identify those with end stage disease.
The best data on the natural history of airflow obstruction in COPD still comes from the study by Fletcher and colleagues.12 The key findings were that COPD developed in a proportion of smokers who experienced a more rapid loss of lung function as a result of smoking. There were wide differences in susceptibility to developing obstruction between smokers, and in the effects of quitting smoking on slowing the annual decline in FEV1. Breathlessness was the symptom associated with greatest loss of lung function and poorest prognosis. Recent work describing a single self-report survey demonstrated a significant strong predictive value of respiratory symptoms for mortality from obstructive lung disease over a 30-year period.13 The Lung Health Study has shown that the accelerated loss of lung function in smokers continues in patients with mild to moderate COPD. Stopping smoking returns the rate of loss of lung function to the value seen in non-smokers14 and is the single most effective way of altering the outcome in patients at all stages of COPD. Although lung function cannot be regained, even those with more advanced disease will benefit.14 However, in end stage COPD patents may struggle to stop smoking. In those dying of the disease it is probably unreasonable to continue promoting smoking cessation. However, by this stage many patients have lost the desire to smoke.
There is surprisingly little information on what patients with COPD die of. Information collected on the causes of death in patients on long-term oxygen therapy showed that about 30% died of acute on chronic respiratory failure. Heart failure was the next most common cause of death (13%), followed by pulmonary infection, pulmonary embolism, cardiac arrhythmia and lung cancer.15 A similar picture was reported over 30 years ago,16 although it is difficult to be sure that all these patients had COPD. Large population studies in Finland, in patients who had had a hospital admission for COPD,17,18 and smaller studies in selected populations19 have found similar causes of death.
Several cross-sectional studies show that there is a clinically significant deterioration of quality of life in COPD.20 Quality of life is impaired even in patients with mild disease, including those in whom there was minimal breathlessness unless undertaking strenuous exercise. Data from the control arms of long-term studies of inhaled steroids in COPD have shown that health related quality of life worsens over time in patients with moderate/severe COPD.21
PROGNOSIS AND ILLNESS TRAJECTORIES
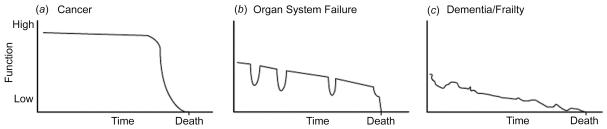
Prognosis has traditionally been the key to the allocation of palliative care services, as evidenced by the eligibility criteria for Medicaid in the USA and the Disability Living Allowance in the UK. These allow a patient expected to live less than six months accelerated benefits to help during this last phase of life. For patients with a cancer diagnosis the issue of prognosis is usually better defined than for patients with other life threatening illnesses such as heart failure and COPD. Over the past few years the concept of illness trajectories has been developed,22 building on the work by Joanne Lynn.23 Lynn describes three recognizable illness trajectories of function and well-being over time: that for cancer a high level of function with a short period of evident decline during which specialist palliative care input would be appropriate; that for organ system failure with long term limitation of function and well-being with intermittent serious episodes sometimes requiring emergency hospital admissions and perhaps a rather sudden death; that for dementia/stroke or frailty marked by low level functioning and prolonged decline with associated problems of providing comprehensive and coordinated services (Figure 1). The physical, social, psychological and spiritual needs of patients and their carers are likely to vary considerably according to the trajectory they are following.22
Figure 1.
General trajectories of function and well-being over time in eventually fatal chronic illness. Reproduced with permission from Lynn.23 JAMA 2001. ;285:925 -32. © 2001 American Medical Association. All Rights reserved.
COPD fits well into the ‘organ system failure’ trajectory, with declining function punctuated by acute exacerbations often triggered by bacterial and viral infections as well as environmental factors. Frequent exacerbations are associated with more rapid decline in FEV1 and worse health status. More severe exacerbations require hospitalization. Mortality after such admissions is high, with rates at three months of 16-19% and at 12 months of 22-43%.24-26 Admission to an intensive care unit was associated with even higher mortality, with 24% dying in hospital.27
Other poor prognostic factors for patients with COPD are increasing age and declining FEV1 together with cardiovascular problems and weight loss.15,28-34
The ever increasing population of patients with heart failure, COPD and stroke and dementias has led Stewart and McMurray to describe ‘prognostic paralysis’.35 ‘Prognostic paralysis’ results when clinicians faced with uncertain illness trajectories prevaricate when considering end-of-lie issues. This can mean that the patients miss out on receiving end-of-lie care progressing to palliative care.36 One way around this problem has been for the clinician to pose the question ‘would I be surprised if my patient were to die in the next 12 months?’ For patients in whom the answer is no, delivery of patient centred active treatment and supportive care are needed and consideration given to discussing end-of-life issues.
ADDRESSING INFORMATION NEEDS
Patients need to be given honest and clear information about the progressive nature of COPD. Many patients report that they have not received enough information about the prognosis and future management37 and there is often a perception that they are receiving mixed messages from different health professionals. Most patients do not want to receive detailed information that they perceive would be distressing, and seem to prefer broad indications of prognosis.37 One reviewer states that patients with far advanced disease are often receptive to the recommendation of a dual agenda: ‘Hope for and expect the best, and prepare for the worst.’38 Work from the USA illustrated the complexity of communication in end-of-life care. Although many barriers and facilitators to end-of-life care discussions were identified it was concluded that it was necessary to identify specific factors from individual patient-physician pairs.39 Clearly there is a challenge for clinicians in striking the right balance for each individual patient between motivating good self-care and causing distress. This is an area where more research is needed.
The treatments that are available to manage advanced disease, including mechanical ventilation, intubation and palliative care strategies, should also be discussed. Many physicians are reluctant to discuss intubation and ventilation with patients.40 This sometimes leads to inappropriate and unwanted treatment when patients are admitted with a severe exacerbation. They are then too ill to be able to express their wishes, resulting in decisions being made by physicians, family members or surrogates. A recent study of attitudes of patients with severe COPD to artificial ventilation and cardiopulmonary resuscitation indicated that such issues can be discussed without causing distress and that attitudes to resuscitation cannot be predicted from parameters of respiratory distress, severity or age.41
How proactive should clinicians be when discussing these issues? A New Zealand study of GP discussion of prognosis42 yielded strategies for use in general practice, which could be extended to other settings. Strategies included awareness of the implications of a COPD diagnosis; use of uncertainty to ease discussion; building relationships over time with patients; being caring and respectful; beginning discussion early in the disease course; identifying and using opportunities to discuss prognosis and working as a team. As well as information about their disease and treatment options, patients need information about what financial and social support is available.43
A recent study of the needs of patients dying from COPD44 indicated that patients desired better education and information about their condition. However, information needs were variable and patients were sometimes unwilling to contemplate the future. The importance of patient education has been demonstrated in a qualitative study from the USA.45 In this study, advance care planning described the communication of diagnosis and disease process, treatment, prognosis and what dying might be like. This was identified as being important to patients with severe COPD. Advance care planning has been implemented in the USA46,47 with some positive outcomes in terms of patient satisfaction, increased incidence of deaths at home and use of hospice services.
How such initiatives might transfer to primary care settings in other countries is uncertain. A UK study indicated that although the majority of GPs acknowledged a need to discuss prognosis in severe COPD, this was not reflected in the reported behaviour. It appeared that the palliative care approach of open communication, while seen to be relevant to severe COPD, is not applied routinely in managing the disease in primary care.48 This may in part be explained by the phenomenon of ‘response shift’, the process whereby in the face of severe disease or impending death, individuals abandon their usual ‘roadmap’ of values and adapt new perspectives.49
Response shifts can make the strict application of advance planning and especially that of living wills or advance directives inappropriate. Palliative care physicians and GPs are particularly well aware of this phenomenon and it may explain their somewhat negative attitude to recording patient's wishes and to long-term planning of specific care. An alternative to the prescriptive advance directive is to make a distinction between a ‘contract’ and a ‘covenant’. The covenant approach involves discerning the patient's core values and acting upon them whilst understanding that there may be changes in some areas such as place of death. The contract approach implies a strict adherence to a specified living will.50 Clearly, further work is required to help narrow the difference between patients' wishes and what actually happens at the end of life.
MANAGING SYMPTOMS AND DISABILITY
Symptom burden
Once it is clear that a patient is in the terminal stages of their disease, adequate symptom palliation is essential. The emphasis of care is often on preventing hospitalization, but these efforts may neglect day-to-day symptoms, with the result that patients live with high levels of symptoms.43 The UK Regional Study of Care for the Dying identified that patients with chronic lung disease at the end of life had physical and psychological needs at least as severe as patients with lung cancer.51 Symptoms of breathlessness were recorded in 94% of chronic lung disease cases, anorexia in 67% and constipation in 44% of cases. Further studies44,52-55 have confirmed the predominance of these symptoms along with significant problems with fatigue, pain, anxiety and panic, poor sleep and depression. A prospective comparison between patients with end stage COPD and lung cancer37 indicated that patients with COPD had significantly worse activities of daily living and physical, social and emotional functioning than patients with lung cancer. In this study 90% of COPD patients suffered clinically relevant anxiety or depression. Sadly none of the patients with COPD received specialist palliative care input.
Managing dyspnoea
The problem of refractory dyspnoea is common and hard to treat. By the time they reach the point of needing palliative care most patients will be receiving maximal bronchodilator therapy and should have received pulmonary rehabilitation. If they have not they should be prescribed a combination of a long-acting beta agonist, an inhaled steroid and a long acting anticholinergic. Theophylline may be of value in some patients, but has a narrow therapeutic index and multiple interactions which may limit its use (Box 2).56 Most patients in the final stages of their illness will be too severely limited to benefit from pulmonary rehabilitation57 and would find it too difficult to attend programmes even if these are run in the community.58 The use of a fan to ease the sensation of dyspnoea is a reasonable option for patients with some supporting evidence of effectiveness in normal subjects.59 Other non-pharmacological interventions may be offered such as relaxation techniques, humidification of air and an unobstructed view. Although evidence for such interventions is weak they may increase patient mastery.
Box 2 Drug therapy of COPD in palliative care
Benzodiazepines to control anxiety
Antidepressants to improve mood
Opioids and oxygen to control breathlessness
Consider continuous subcutaneous infusion therapy of opioids, anti-emetics and anxiolytics
A part from the optimizing of medical treatment, oxygen and opioids remain the mainstays of palliative pharmacological treatment. There is as yet no evidence of the effectiveness of oxygen in the palliation of breathlessness (see below). This is due in part to the lack of randomized controlled trials using reduction of breathlessness as an outcome measure. The conclusion of the Association of Palliative Medicine working group was that oxygen use has to be tailored to the individual and a formal assessment made of its efficacy for reducing breathlessness and improving quality of life for that person.60 Use of opioids in the management of dyspnoea has become clearer. A systematic review61 supported the continued use of oral and parenteral opioids to treat dyspnoea in advanced disease. This also provided reassurance that appropriate doses of opioids do not lead to respiratory depression. The usefulness of nebulized opioids is unclear, but it appears that they are no better than nebulized normal saline. More specific data regarding the use of oral opioids for managing refractory dyspnoea in patients with predominantly COPD have been recently published.62 In this study a slow release preparation of 20 mg of morphine sulphate given once daily provided significant symptomatic improvement in refractory dyspnoea in the community setting. Subsequent availability of lower strength sustained release morphine preparations allows dose titration for the opioid naive patient with COPD.
Oxygen therapy
As the COPD progresses patients often become hypoxaemic. Many patients tolerate mild hypoxaemia well, but once the resting PaO2 falls below 8 kPa patients begin to develop signs of cor pulmonale, principally peripheral oedema. Once this occurs the prognosis is poor and if untreated the five-year survival is less than 50%.
In patients with end stage disease, oxygen is used to provide symptomatic relief of breathlessness. Oxygen is normally used with caution in patients with COPD as uncontrolled oxygen therapy can result in suppression of respiratory drive, carbon dioxide narcosis and ultimately respiratory arrest in some patients with COPD.
Oxygen can be administered for long periods during the day and night (long term oxygen therapy) or as short burst therapy to relieve symptoms. Long term oxygen therapy (15 hours a day) aims to improve survival in patients with COPD who have severe hypoxaemia (PaO2 <8 kPa)63 as well as reducing the incidence of polycythaemia, reducing the progression of pulmonary hypertension and improving neuropsychological health. Apart from the latter, these are not generally important issues in patients receiving palliative care. An oxygen concentrator is the most cost effective mode of delivery of long term oxygen therapy.
Short burst oxygen is commonly prescribed for use by patients who do not meet the criteria for long term oxygen therapy but who remain breathless after minimal exertion despite other therapy. It is usually provided from cylinders. Patients report considerable symptomatic benefit and earlier recovery after exercise with short-burst oxygen, though there is little evidence to support this finding and the effects may not be reproducible with time.64 One study showed that patients with chronic hypoxaemia due to COPD or interstitial lung disease show a reduction in dyspnoea after 10 minutes of supplemental oxygen therapy, though normoxaemic patients were not studied.65 Short term ambulatory oxygen use was associated with significant improvement in health-related quality of life of COPD patients.66 However, a recent randomized controlled trial comparing short-burst oxygen therapy with cylinder air or usual care after an acute exacerbation revealed no significant improvement in health related quality of life or reduction in acute health care utilization in the short burst oxygen group.67
The NICE guideline recommends that short burst oxygen therapy should only be considered for episodes of severe breathlessness in patients with COPD not relieved by other treatments. It should only continue to be prescribed if an improvement in breathlessness following therapy has been documented, and when indicated it should be provided from cylinders. If oxygen is required then caution with continuing smokers is necessary as there is a real risk of fire, explosion or facial burns.68
Weight loss and muscle wasting
Weight loss and associated muscle wasting is a common feature in patients with severe COPD and the pathophysiology is incompletely understood.69 Interventions have centred on calorie and protein supplementation, with limited success. However, when supplementation is used in conjunction with exercise, increases in body weight and respiratory muscle strength have been observed, especially when delivered in the context of a pulmonary rehabilitation programme,70 but this may not be feasible for patients dying with COPD.
Poor appetite and weight loss are of concern to the patient and carer alike and need to be taken seriously by the community team. The advice of a dietician may be beneficial in terms of support if not in actual weight gain.70
Managing anxiety and depression
Anxiety associated with dyspnoea is commonplace. Drug therapy with benzodiazepines is commonly used in clinical practice, but it is hard to find published evidence for safety and effectiveness. From personal experience, judicious use of low doses of diazepam can help reduce anxiety. Likewise insomnia is commonly reported in COPD and yet evidence for the use of benzodiazepines for insomnia in palliative care is not yet available.71 A review of anxiety and self-management behaviour in COPD illustrates the difficulties of transferring self-management techniques employed successfully in asthma patients to patients with COPD. It appears that in some cases anxiety can be compounded by the very information being imparted in the self-management programme.72
Depressive illness is commonly reported in patients dying from cancer and it is considered good practice to actively seek this diagnosis and actively treat the condition. The same should apply to those suffering from COPD. Even prior to the terminal phase, COPD is strongly associated with anxiety and depression—particularly in those who are hypoxic or severely dyspnoeic.73 The symptoms are disabling and distressing, and patients often become socially isolated and have to give up activities that they enjoy. The psychosocial effects of the disease may be reinforced by the depressed mood. The NICE guideline on COPD recommends that patients who are found to be depressed or anxious should be treated with conventional pharmacotherapy, but for antidepressant treatment to be successful, it needs to be supplemented by spending time with the patient explaining why depression needs to be treated alongside the physical disorder.1
Towards the end of life the oral route of administration may become inappropriate and continuous subcutaneous infusion therapy via a syringe driver should be considered.
Co-morbidities at the end of life
The issue of managing co-morbidities in patients at the end of life is growing in importance and helpful advice has recently been published.74 Whether or not to continue regular medication for conditions such as arthritis, hypertension, ischaemic heart disease, atrial fibrillation (with anticoagulation) and diabetes requires active consideration by the GP and careful discussion with the patient.
The place of complementary and alternative medicine
There is limited evidence for the use of complementary and alternative medicine in the management of dyspnoea.75 Individual patients with severe COPD may benefit from the use of acupuncture, acupressure and muscle relaxation with breathing retraining to relieve dyspnoea,76 though no benefit from acupuncture was demonstrated in a randomized controlled trial.77 As these interventions are low risk it would seem reasonable to employ these therapies on an individual basis if they are requested by the patient.
IMPROVING THE PATIENT EXPERIENCE OF COPD
As already discussed, the duration of the illness and uncertain prognosis places great strain on patient and carers alike. This is typified by a lack of surveillance and inadequate services from secondary and primary care in the year before death.54 Qualitative studies indicate that patients experience losses of personal liberty and dignity and of previously held expectations of the future.52 However, adaptive strategies to cope with the effects of the disease can emerge over the lengthy period of time patients and carers live with the disease. Relationships with health care professionals may mediate the development of adaptive strategies, but these can be both positive and negative. In particular, patients appreciate continuity of care and reassurance provided by the primary health care team.52,78
The role of secondary care is viewed in a number of ways. Some patients and carers appreciate the surveillance role carried out by secondary care clinics, someone to check up on their condition and ‘keep an eye on them’. Others find the whole experience of attending hospital too exhausting, with the consultation perfunctory and lacking in continuity.
Paying attention to carer's health needs is relevant. Carers suffer significantly, experiencing severe anxiety and helplessness as they witness the patient's suffering and experience severe restrictions to their own lifestyle.54 Carers become enmeshed with the patient's illness and often take on numerous roles ‘we're nurses, we're doctors, we're housewives, we're cooks, we're gardeners.’52 The plethora of roles extending sometimes over years takes its toll on carers in terms of exhaustion and mental health problems.
WHAT DO PATIENTS WITH END STAGE COPD WANT?
The majority of patients with life-threatening illness express the wish to die at home, though a substantial minority may express a wish to die in other settings, such as an inpatient hospice.79 Where possible it is reasonable to try and explore an individual's wishes. Whilst it is not always possible to die at home, it is reasonable to aim for the major part of the final phase of life to be spent at home. Most patients with severe COPD are initially receiving secondary care services and this can take the form of outpatient clinics and in some cases input from respiratory nurse specialists. The role of the specialist nurse has been shown to improve satisfaction with care, and improved patient education, but had no effect in reducing acute hospital admissions for exacerbations.80 However, specialist outreach respiratory nursing is as yet of unproven benefit in patients with severe disease and those who are dying.81 Some patients and carers will benefit from the reassurance and the surveillance that outpatient clinics and specialist nurses can provide. However, there will come a point for many when community based care with a predominant palliative element is more appropriate. Judging this transition point requires experience, sensitivity and open communication from and between both the hospital clinician and general practitioner. Having reached this point it is essential that the patient and carer do not get lost to follow up in the community, and the planning of long-term support is important. It has been observed that patients are often reluctant to fully express their needs and may be reluctant to ‘bother the doctor,’ and so the elicitation of patient and carer needs and preferences requires some sensitivity.78 In the UK the implementation of the Gold Standards Framework for palliative care is proceeding.82 This initiative seeks to help primary health care teams put in place structures for effective palliative care for patients with life threatening illness in the community. Although originally developed for patients with cancer, it should be possible to adapt the recommendations to the needs of patients with cardiopulmonary disease.
Most hospice services in the UK accept patients with non-malignant illness and this openness should increase with the recent publication of NICE guidelines, which encourages a palliative care approach for patients with severe COPD.1 There is a growing emphasis on involving hospice services at an earlier stage for patients with a cancer diagnosis. For patients with cardiopulmonary disease the issue needs to be discussed sensitively with the patients, with the emphasis on the positive aspects of being able to help with symptom control, arrange community support, provide relevant information and act as a sympathetic listener in person or at the end of the telephone.
Care of the dying patient has been recently reviewed, with emphasis on evidence-based guidelines on symptom control, psychological support and bereavement allowing facilitation of a ‘good’ death.83 Particular note is made of the importance of diagnosing dying. This is a challenge for patients with COPD and as noted above, the death of such patients often occurs with an exacerbation and can appear sudden and unexpected. The prognosis related to reduced FEV1 and the requirement for long term oxygen therapy, as described earlier, can act as a guide to when to commence palliative care. Certainly the expectation of death within the next 12 months is a strong pointer to adopting a palliative approach (Box 3).
Box 3 Features related to poor prognosis in COPD
Severe airflow obstruction <30% FEV1
Frequent exacerbations
Requirement for long-term oxygen therapy
Clinician's expectation of death in the next 12 months
Development of cor pulmonale
The place of death may not be the home. Severe exacerbations of COPD can be distressing for patient and carer alike and beyond the skills and resources of the community team to deal with. For patients expressing a wish not to go into a general hospital the options are to stay at home or to consider hospice admission or admission to a local facility such as a GP-run community hospital. The community hospital has advantages of care being provided by the patient's own GP, proximity to home and often staff known to the patient.84,85
CONCLUSION
COPD is a disease with a lengthy illness trajectory and high level of symptom burden associated with psychosocial effects on patients and their families. The management of the patients should be shared between primary and secondary care, with multidisciplinary teams being involved at an early stage in the disease. Judging the transition from active treatment to supportive palliative care can be difficult. However, severely impaired respiratory function, requirement for long term oxygen therapy and frequent exacerbations are indicators that this stage may have been reached. Patients and their families require honest and clear communication about the condition and what to expect in the future. Advance care planning (including living wills and advance directives) has a place, but may not need to take the form of a written agreement. However, it is important that patients and their carers are given the opportunity to share their concerns and wishes for future care with the team caring for them, and that this information is shared and recorded.
The evidence base for symptom control in COPD is poor and management relies on experience and considered best practice. However, there is evidence for judicious use of opiates and oxygen in the treatment of dyspnoea. Specialist outreach respiratory nursing is as yet of unproven benefit, but patients and their carers appear to appreciate the reassurance and surveillance role outreach nurses can provide. The hospice movement has become increasingly involved in the management of life threatening non-malignant disease and should be involved in the multidisciplinary care of patients dying from COPD.
Competing interests None declared.
Funding The Honiton Research Practice is a NHS Funded Research Practice.
Acknowledgments Thanks are due to Colin Greaves for his helpful comments on the manuscript.
References
- 1.National Institute for Clinical Excellence (NICE). Chronic obstructive pulmonary disease. National clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004. ;59(Suppl 1):1 -232 [PMC free article] [PubMed] [Google Scholar]
- 2.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256 -76 [DOI] [PubMed] [Google Scholar]
- 3.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932 -46 [DOI] [PubMed] [Google Scholar]
- 4.Health and Social Care Information Centre. Statistical Bulletin 2005/02/HSCIC. National Quality and Outcomes Framework Statistics for England 2004/05. London: Health and Social Care Information Centre, 2005
- 5.Soriano JB, Maier WC, Egger P, et al. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax 2000. ;55:789 -94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannino DM, Brown C, Giovino GA. Obstructive lung disease deaths in the United States from 1979 through 1993. An analysis using multiple-cause mortality data. Am J Respir Crit Care Med 1997. ;156:814 -8 [DOI] [PubMed] [Google Scholar]
- 7.Office for National Statistics. Mortality statistics: Cause, 1999. DH2(No 26). London: HMSO;2000
- 8.Office for National Statistics. Health Statistics Quarterly. (8) Winter 2000. London: HMSO;2000
- 9.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2000;160:1683 -9 [DOI] [PubMed] [Google Scholar]
- 10.Wolkove N, Dajczman E, Colacone A, Kreisman H. The relationship between pulmonary function and dyspnea in obstructive lung disease. Chest 1989. ;96:1247 -51 [DOI] [PubMed] [Google Scholar]
- 11.Halpin DMG. Assessing the severity of COPD. Prim Care Respir J 2006;15:78 -80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ 1977. ;1:1645 -8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frostad A, Soyseth V, Haldorsen T, Andersen A, Gulsvik A. Respiratory symptoms and 30 year mortality from obstructive lung disease and pneumonia. Thorax 2006. ;61:951 -6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med 2000;161:381 -90 [DOI] [PubMed] [Google Scholar]
- 15.Zielinski J, MacNee W, Wedzicha J, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis 1997;52:43 -7 [PubMed] [Google Scholar]
- 16.Burrows B, Earle RH. Course and prognosis of chronic obstructive lung disease. A prospective study of 200 patients. N Engl J Med 1969;280:397 -404 [DOI] [PubMed] [Google Scholar]
- 17.Keistinen T, Tuuponen T, Kivela SL. Survival experience of the population needing hospital treatment for asthma or COPD at age 50-54 years. Respir Med 1998. ;92:568 -72 [DOI] [PubMed] [Google Scholar]
- 18.Vilkman S, Keistinen T, Tuuponen T, Kivela SL. Survival and cause of death among elderly chronic obstructive pulmonary disease patients after first admission to hospital. Respiration 1997. ;64:281 -4 [DOI] [PubMed] [Google Scholar]
- 19.Nishimura K, Tsukino M. Clinical course and prognosis of patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med 2000;6:127 -32 [DOI] [PubMed] [Google Scholar]
- 20.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992. ;145:1321 -7 [DOI] [PubMed] [Google Scholar]
- 21.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000. ;320:1297 -303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ 2005. ;330:1007 -11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynn J. Perspectives on care at the close of life. Serving patients who may die soon and their families: the role of hospice and other services. JAMA 2001. ;285:925 -32 [DOI] [PubMed] [Google Scholar]
- 24.Hosker HSR, Anstey K, Lowe D, Pearson M, Roberts MC. The outcome of patients with COPD admitted to UK hospitals with an exacerbation. Results from the RCP/BTS national COPD audit. Eur Respir J 2005. ;26(Suppl 49):463s [Google Scholar]
- 25.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003. ;124:459 -67 [DOI] [PubMed] [Google Scholar]
- 26.Eriksen N, Hansen EF, Munch EP, Rasmussen FV, Vestbo J. [Chronic obstructive pulmonary disease. Admission, course and prognosis]. Ugeskr Laeger 2003. ;165:3499 -502 [PubMed] [Google Scholar]
- 27.Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959 -67. Erratum appears in [DOI] [PubMed] [Google Scholar]; Am J Respir Crit Care Med 1997. ;155:386 [Google Scholar]
- 28.Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986. ;133:14 -20 [DOI] [PubMed] [Google Scholar]
- 29.France AJ, Prescott RJ, Biernacki W, Muir AL, MacNee W. Does right ventricular function predict survival in patients with chronic obstructive lung disease? Thorax 1988. ;43:621 -6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonelli Incalzi R, Fuso L, De Rosa M, et al. Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J 1997. ;10:2794 -800 [DOI] [PubMed] [Google Scholar]
- 31.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999. ;160:1856 -61 [DOI] [PubMed] [Google Scholar]
- 32.Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002. ;166:809 -913 [DOI] [PubMed] [Google Scholar]
- 33.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998. ;157:1791 -7 [DOI] [PubMed] [Google Scholar]
- 34.Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J 2002;20:539 -44 [DOI] [PubMed] [Google Scholar]
- 35.Stewart S, McMurray JJ. Palliative care for heart failure. BMJ 2002. ;325:915 -6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray SA, Boyd K, Sheikh A. Palliative care in chronic illness. BMJ 2005. ;330:611 -2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax 2000. ;55:1000 -6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care 2004. ;49:90 -8 [PubMed] [Google Scholar]
- 39.Knauft E, Nielsen EL, Engelberg RA, Patrick DL, Curtis JR. Barriers and facilitators to end-of-life care communication for patients with COPD. Chest 2005. ;127:2188 -96 [DOI] [PubMed] [Google Scholar]
- 40.Sullivan KE, Hebert PC, Logan J, O'Connor AM, McNeely PD. What do physicians tell patients with end-stage COPD about intubation and mechanical ventilation? Chest 1996. ;109:258 -64 [DOI] [PubMed] [Google Scholar]
- 41.Gaber KA, Barnett M, Planchant Y, McGavin CR. Attitudes of 100 patients with chronic obstructive pulmonary disease to artificial ventilation and cardiopulmonary resuscitation. Palliat Med 2004. ;18:626 -9 [DOI] [PubMed] [Google Scholar]
- 42.Halliwell J, Mulcahy P, Buetow S, Bray Y, Coster G, Osman LM. GP discussion of prognosis with patients with severe chronic obstructive pulmonary disease: a qualitative study. Br J Gen Pract 2004. ;54:904 -8 [PMC free article] [PubMed] [Google Scholar]
- 43.Skilbeck J, Mott L, Page H, Smith D, Hjelmeland-Ahmedzai S, Clark D. Palliative care in chronic obstructive airways disease: a needs assessment. Palliat Med 1998. ;12:245 -254 [DOI] [PubMed] [Google Scholar]
- 44.Jones I, Kirby A, Ormiston P, et al. The needs of patients dying of chronic obstructive pulmonary disease in the community. Fam Pract 2004. ;21:310 -3 [DOI] [PubMed] [Google Scholar]
- 45.Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Patients' perspectives on physician skill in end-of-life care: differences between patients with COPD, cancer, and AIDS. Chest 2002. ;122:356 -62 [DOI] [PubMed] [Google Scholar]
- 46.Tierney WM, Dexter PR, Gramelspacher GP, Perkins AJ, Zhou XH, Wolinsky FD. The effect of discussions about advance directives on patients' satisfaction with primary care. J Gen Intern Med 2001. ;16:32 -40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammes BJ, Rooney BL. Death and end-of-life planning in one midwestern community. Arch Intern Med 1998. ;158:383 -90 [DOI] [PubMed] [Google Scholar]
- 48.Elkington H, White P, Higgs R, Pettinari CJ. GPs' views of discussions of prognosis in severe COPD. Fam Pract 2001. ;18:440 -4 [DOI] [PubMed] [Google Scholar]
- 49.Schwartz C. Decision making at the end of life: shifting sands. J R Soc Med 2005. ;98:297 -8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fins JJ, Maltby BS, Friedmann E, et al. Contracts, covenants and advance care planning: an empirical study of the moral obligations of patient and proxy. J Pain Symptom Manag 2005. ;29:55 -68 [DOI] [PubMed] [Google Scholar]
- 51.Edmonds P, Karlsen S, Khan S, Addington-Hall J. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat Med 2001. ;15:287 -95 [DOI] [PubMed] [Google Scholar]
- 52.Seamark DA, Blake SD, Seamark CJ, Halpin DM. Living with severe chronic obstructive pulmonary disease (COPD): perceptions of patients and their carers. An interpretative phenomenological analysis. Palliat Med 2004;18:619 -25 [DOI] [PubMed] [Google Scholar]
- 53.Elkington H, White P, Addington-Hall J, Higgs R, Pettinari C. The last year of life of COPD: a qualitative study of symptoms and services. Respir Med 2004. ;98:439 -45 [DOI] [PubMed] [Google Scholar]
- 54.Elkington H, White P, Addington-Hall J, Higgs R, Edmonds P. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med 2005. ;19:485 -91 [DOI] [PubMed] [Google Scholar]
- 55.Booth S, Silvester S, Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: using a qualitative approach to describe the experience of patients and carers. Palliat Support Care 2003;1:337 -44 [DOI] [PubMed] [Google Scholar]
- 56.Aronson JK, Hardman M, Reynolds DJ. ABC of monitoring drug therapy. Theophylline. BMJ 1992. ;305:1355 -8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pulmonary rehabilitation. Thorax 2001. ;56:827 -34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ward JA, Akers G, Ward DG, et al. Feasability and effectiveness of a pulmonary rehabilitation programme in a community hospital setting. Br J Gen Pract 2002. ;52:539 -542 [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartzstein RM, Lahive K, Pope A, Weinberger SE, Weiss JW. Cold facial stimulation reduces breathlessness induced in normal subjects. Am Rev Respir Dis 1987. ;136:53 -61 [DOI] [PubMed] [Google Scholar]
- 60.Booth S, Wade R, Johnson M, Kite S, Swannick M, Anderson H. The use of oxygen in the palliation of breathlessness. A report of the expert working group of the Scientific Committee of the Association of Palliative Medicine. Respir Med 2004. ;98:66 -77 [DOI] [PubMed] [Google Scholar]
- 61.Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax 2002. ;57:939 -944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003. ;327:523 -8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cranston J, Crockett A, Moss, Jr., Alpers J, Cranston J. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005. ;4:CD001744 [DOI] [PMC free article] [PubMed]
- 64.Evans TW, Waterhouse JC, Carter A, Nicholl JF, Howard P. Short burst oxygen treatment for breathlessness in chronic obstructive airways disease. Thorax 1986. ;41:611 -5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swinburn CR, Mould H, Stone TN, Corris PA, Gibson GJ. Symptomatic benefit of supplemental oxygen in hypoxemic patients with chronic lung disease. Am Rev Respir Dis 1991. ;143:913 -5 [DOI] [PubMed] [Google Scholar]
- 66.Eaton T, Garrett JE, Young P, et al. Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled study. Eur Respir J 2002. ;20:306 -12 [DOI] [PubMed] [Google Scholar]
- 67.Eaton T, Fergusson W, Kolbe J, Lewis CA, West T. Short-burst oxygen therapy for COPD patients: a 6-month randomised, controlled study. Eur Respir J 2006. ;27:697 -704 [DOI] [PubMed] [Google Scholar]
- 68.West GA, Primeau P. Nonmedical hazards of long-term oxygen therapy. Respir Care 1983. ;28:906 -12 [PubMed] [Google Scholar]
- 69.Wouters EF. Management of severe COPD. Lancet 2004. ;364:883 -95 [DOI] [PubMed] [Google Scholar]
- 70.Creutzberg EC, Wouters EF, Mostert R, Weling-Scheepers CA, Schols AM. Efficacy of nutritional aupplementation therapy in depleted patients with chronic obstructive pulmonary disease. Nutrition 2003. ;19:120 -7 [DOI] [PubMed] [Google Scholar]
- 71.Hirst A, Sloan R. Benzodiazepines and related drugs for insomnia in palliative care. Cochrane Database Syst Rev 2002. ;4:CD003346 [DOI] [PubMed]
- 72.Dowson CA, Kuijer RG, Mulder RT. Anxiety and self-management behaviour in chronic obstructive pulmonary disease: what has been learned? Chron Respir Dis 2004. ;1:213 -20 [DOI] [PubMed] [Google Scholar]
- 73.van Manen JG, Bindels PJ, Dekker FW, CJ IJ, van der Zee JS, Schade E. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax 2002. ;57:412 -6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stevenson J, Abernethy AP, Miller C, Currow DC. Managing comorbidities in patients at the end of life. BMJ 2004. ;329:909 -12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan CX, Morrison RS, Ness J, Fugh-Berman A, Leipzig RM. Complementary and alternative medicine in the management of pain, dyspnea, and nausea and vomiting near the end of life. A systematic review. J Pain Symptom Manage 2000. ;20:374 -87 [DOI] [PubMed] [Google Scholar]
- 76.Corner J, Plant H, A'Hern R, Bailey C. Non-pharmacological intervention for breathlessness in lung cancer. Palliat Med 1996;10:299 -305 [DOI] [PubMed] [Google Scholar]
- 77.Lewith GT, Prescott P, Davis CL. Can a standardized acupuncture technique palliate disabling breathlessness: a single-blind, placebo-controlled crossover study. Chest 2004. ;125:1783 -90 [DOI] [PubMed] [Google Scholar]
- 78.Exley C, Field D, Jones L, Stokes T. Palliative care in the community for cancer and end-stage cardiorespiratory disease: the views of patients, lay-carers and health care professionals. Palliat Med 2005;19:76 -83 [DOI] [PubMed] [Google Scholar]
- 79.Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med 2000;3:287 -300 [DOI] [PubMed] [Google Scholar]
- 80.Hermiz O, Comino E, Marks G, Daffurn K, Wilson S, Harris M. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ 2002. ;325:938 -42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor SJ, Candy B, Bryar RM, et al. Effectiveness of innovations in nurse led chronic disease management for patients with chronic obstructive pulmonary disease: systematic review of evidence. BMJ 2005. ;331:485 -8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas K. Caring for the Dying at Home. Companions on a Journey. Oxford: Radcliffe Medical Press,2003
- 83.Ellershaw J, Ward C. Care of the dying patient: the last hours or days of life. BMJ 2003. ;326:30 -4 [PMC free article] [PubMed] [Google Scholar]
- 84.Seamark DA, Williams S, Hall M, Lawrence CJ, Gilbert J. Dying from cancer in community hospitals or a hospice: closest lay carers' perceptions. Br J Gen Pract 1998. ;48:1317 -21 [PMC free article] [PubMed] [Google Scholar]
- 85.Payne S, Kerr C, Hawker S, et al. Community hospitals: an under-recognized resource for palliative care. J R Soc Med 2004;97:428 -31 [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional Reading
- Admedzai SH, Muers MM eds: Supportive care in respiratory disease. Oxford OUP 2005. ISBN: 0-19-263141-1.
- Wouters EFM. Management of severe COPD. Lancet 2004. ;364:883 -95 [DOI] [PubMed] [Google Scholar]