Abstract
Objective We describe our experience of using the standard application form, designed to streamline applications for multi-centre research, to seek approval from all primary care organizations (PCOs) in England and Wales to undertake a single telephone interview with a health service manager.
Design We sent applications (n=316), by email to each PCO, or consortium of PCOs, attaching a completed standard application form, the 15 required documents, and the approval we had been granted by the lead NHS organization. We maintained detailed records of the responses to our application, subsequent correspondence, additional paperwork requested, and time spent on the approval process.
Setting The UK Research Governance Framework, which regulates all research conducted in health and social care settings.
Participants All PCOs in England and Wales.
Interventions None.
Main outcome measures Time taken to obtain approval to undertake a telephone interview with a health service manager.
Results We were unable to establish contact with 13 (4%) PCOs. Six months after submitting our application we had received approval from 259/316 (82%) PCOs and were still awaiting a verdict from 41 (13%). The median time to approval was 56 days (IQR 42-72). Overall, an estimated 318 staff-hours were spent completing supplementary forms, providing additional information and chasing up dormant applications.
Conclusions Recent initiatives to ‘streamline’ research governance approval have failed to address the problems that face researchers undertaking multi-centre studies. There is an urgent need to develop a simpler process that allows low risk research to take place without threatening staff morale and endangering the quality of the research outputs. In the meantime, we advise researchers to allow far greater time than might reasonably be envisioned to obtain research governance approval.
INTRODUCTION
Against a background of increasing global concern about fraud and misconduct in medical research,1 the Department of Health introduced a research governance framework in 2001 to regulate all research conducted in health and social care settings.2,3 Official systems were introduced, embodying a broad range of regulations, principles and standards of good research practice that aimed to improve research quality and accountability across all aspects of health care. Responsibility for implementing the framework was devolved to local primary care organizations (PCOs) and/or hospital trusts. Organizations in England are encouraged to collaborate within research and development (R&D) consortia;4 similarly, in Wales, the Capricorn Research Network grants R&D approval for all Welsh PCOs. An ‘information toolkit’ has been developed by the NHS R&D Forum (http://www.rdforum.nhs.uk) which draws together practical examples of approval processes to assist PCOs in meeting their research governance obligations.5
Recent literature on the R&D approval process has reflected on the rationale behind research governance procedures,1 explored how the governance process judges the risk of harm within research6 and described the underlying ethical principles,7,9 whilst arguing for flexibility in the application of these principles to health care research.6,7,10 While the justification for governance (‘to ensure that the public can have confidence in, and benefit from, quality research in health and social care’2) is clear, several researchers have publicly expressed frustration and demoralization resulting from stringent and lengthy research governance procedures that impede the onset, progress, quality and outcomes of research.11-16 In response to these concerns, a standard R&D application form (Form D) has recently been introduced to streamline applications for multi-centre research which ‘all NHS organizations are strongly recommended to accept.’17
This paper is the first to describe the experience of using the new ‘streamlined’ system to apply to all PCOs in England and Wales for R&D approval for the initial phase of an ethnographic study exploring respiratory workforce dynamics in PCOs in England and Wales.18 This first phase involved conducting a single telephone interview (maximum duration 45 minutes) with a health service manager in a sample of PCOs with a range of demographic profiles and offering diverse approaches to the delivery of respiratory services. Our project schedule required us to complete these interviews over three months, in preparation for subsequent in-depth case studies of the development of respiratory care in selected PCOs. Since approval was sought simultaneously from all PCOs in England and Wales, our experience provides a national snapshot of the current state of obtaining governance approval for low-risk, multi-centre research.
METHODS
Ethics approval and R&D approval from a lead NHS organization
Ethics approval was granted on 8 December 2005, three weeks in advance of the start of the study. We opted to request governance approval from all 325 PCOs in England and Wales so that we could establish a pool of PCOs from which to sample organizations with a broad range of geographic, demographic and service profiles. Following the guidance on the R&D Forum website,19 we discussed our application with a lead NHS organization, whose R&D manager provided helpful advice and granted approval for the nine PCOs within the local R&D consortium on 18 January 2006.
Approval from the 316 remaining PCOs in England and Wales
During the week beginning 24 January 2006, we sent out applications by email to 76 R&D leads covering 310 of the remaining 316 PCOs in consortiums or individually, as listed on the R&D Forum website for England and Wales. No contact details were available for the R&D leads in the other six PCOs. We attached a completed standard R&D application form (Form D) and the 15 required documents (Table 1). In addition, we appended the approval we had been granted by the lead NHS organization.
Table 1.
Documents accompanying the initial R&D approval applications
| Document number | Description |
|---|---|
| 1 | NHS REC Application Form |
| 2 | NHS R&D Application Form |
| 3 | Scan of Signed R&D Form |
| 4 | Project Proposal |
| 5 | Project Flow Diagram |
| 6 | Screening Interview Topic Guide |
| 7 | Invitation Letter for Screening Interviews |
| 8 | Reminder 1 for Screening Interviews |
| 9 | Reminder 2 for Screening Interviews |
| 10 | Information Leaflet for Screening Interviews |
| 11 | Consent Form for Screening Interviews |
| 12 | Principal Investigator's CV |
| 13 | Sponsor Confirmation |
| 14 | MREC Approval |
| 15 | Lead Organization's R&D Approval |
Record of progress
The study researchers and secretary maintained detailed records of the responses to our applications, subsequent correspondence, and additional paperwork requested. Time spent on the approval process was noted at the end of each working day.
RESULTS
Initial responses
The first response arrived within a day, but the median time for an initial response was 30 days (IQR 22-56). Of the 76 R&D leads contacted, 12 (16%) gave immediate approval either by post or an email, another 12 (16%) acknowledged receipt of the request for approval and provided a timeframe within which they would respond once they had spoken to the relevant committee. It transpired that a further 12 (16%) of our applications had been sent to an outdated or ‘out of office’ R&D contact and were then redirected to the correct individuals. About a third (i.e. 27, 37%) requested further information on the study or had specific requirements for their individual organizations. The requests included queries relating to indemnity, funding, sponsorship, supplementary forms specific to the PCO in question (often duplicating information already provided), XML versions of ethics and R&D forms, additional curriculum vitae and requests for evidence of the peer review process. Three of the Welsh PCOs asked us to complete an alternative form on the Capricorn R&D Network website. Ten R&D leads (13%) had still not replied six months after the initial applications were sent off.
PCO approval granted
The median time to gaining approval was 56 days (IQR 42-72); three PCOs (1%) declined approval. By the end of July 2006 (i.e. six months after submitting our application) we had received approvals from 259/316 PCOs (82%) across England and Wales, although approval for 16 Welsh PCOs was conditional on translating the information sheet and consent form into Welsh. Time constraints have prevented us from complying with this requirement and we have thus lost the opportunity to gain potentially valuable insights into respiratory service development in many of the Welsh PCOs. We had been unable to contact 13 PCOs (4%) (six without contact details, and seven because the details provided were incorrect) and were still awaiting a verdict from 41 (13%) after six months, by which time we had no choice but to move on with subsequent phases of the study.
Time implications and opportunity costs
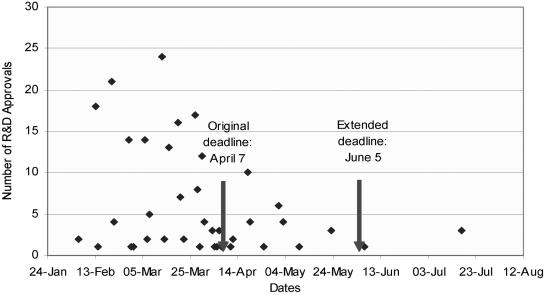
Overall, an estimated 318 staff-hours were spent achieving R&D approval. This occupied all our secretarial time, a substantial proportion of the senior researcher's time and considerable time input from the principal investigator over about 10 weeks, as we were required to complete supplementary forms, reformulate letters and chase up dormant applications, rendering the study about two months behind schedule (Figure 1). The on-going requests for progress reports from the R&D offices, despite our sending a standard report to all R&D offices from whom we had had approval at six months, is currently further delaying the project.
Figure 1.
Time taken to obtain R&D approvals from 259/316 PCOs
DISCUSSION
We have found that obtaining R&D approval for a very low risk, multi-centre descriptive study involving NHS staff remains, despite the new ‘streamlined’ R&D approval procedure, an extremely cumbersome and time-consuming process. Despite planning meticulously for this study and undertaking a good deal of preparatory work before the commencement of the project, these barriers to undertaking the research, which are eminently avoidable, have resulted in serious delays, threatened staff morale and adversely impacted on the time for analysis, reviewing literature and preparing for subsequent phases of the study.
Limitations and strengths of this work
The minimal intervention for which we sought approval represented no foreseeable risk to participants, so our findings may not be representative of the delays imposed by the process on complicated intervention studies, and particularly those involving patients. Also, our data only relate to governance approval in England and Wales, and these experiences may thus not be generalizable to Scotland or Northern Ireland. The study's main strength lies in the fact that we attempted to approach all PCOs in England and Wales either individually or through their R&D consortium. Importantly, it is the first study to describe the experience of using the ‘streamlined’ system.
Interpretation of findings in relation to the published literature
Little appears to have changed since the introduction of a standard R&D application form in March 2005 designed to ‘streamline the R&D approval process’ and ‘minimise unnecessary bureaucracy for researchers.’17,20 The process still feels impossibly cumbersome, taking a median of 56 days for 82% of PCOs to approve a short interview with a health care manager. Our experience is very similar to that reported before the introduction of the standard process. In 2003/04, only 80% of English PCOs had granted approval for a questionnaire survey of health care professionals after four months.13 Another study involving patient questionnaires reported taking 103 days to achieve approval in all the 20 NHS trusts to which they applied.14 A key factor is the devolution of research governance to individual trusts and a lack of central guidance, which has resulted in diverse local interpretation and a plethora of different forms and procedures.5 Although the standard application form is ‘strongly recommended’ by the R&D Forum, it is not mandatory, and supplementary forms and procedures abound. Six months after the introduction of the ‘streamlined’ procedures, a survey of PCO R&D officers found that although they preferred their own local documentation, they stated that they would accept the standard application form.20 Our study, in which a third of organizations requested additional forms or information, suggests that this may not be the case.
Discussion papers have called for flexibility in estimating risk and applying research governance procedures.6,7,10 Our study, in which the nature of the research was harmless enough to show the absurdity of stringent governance requirements, illustrates this need. While it is clear that staff involved in health research owe full accountability to their study participants, governance procedures that have developed in response to investigational medicinal products cannot be appropriately applied to all applications for R&D approval.
The NHS R&D Forum have just released a common form for both local ethical and R&D approval which will hopefully reduce the burden of form filling.21 However, this does not address the key underlying problem of each trust enforcing regulations according to their own particular interpretation of the responsibilities. A system in which the lead R&D department (or a ‘Multicentre R&D committee’) classified research as being of high, medium or low risk according to agreed criteria, so that local trusts would then apply uniform regulations appropriate to the research, would seem sensible. Timescales, such as apply to ethics applications, should be mandatory.
CONCLUSIONS
Recent initiatives to ‘streamline’ research governance approval have failed to address the problems that face researchers undertaking multi-centre studies. There is an urgent need for R&D departments, researchers and research funders to cooperate in developing a simpler process that allows low risk research to take place without threatening the morale of staff and endangering the viability and quality of the research project. The ‘bureaucracy busting’ intentions and plans for a research passport system as part of the DoH ‘Best Research for Best Health’ strategy offer some hope for the future.22 In the meantime, in the light of our experience, we advise researchers to allow ample time to obtain R&D approval and ask funding bodies to be sympathetic to the associated unacceptable, but at present unavoidable, delays and associated increased costs.
Competing interests None declared.
Funding NHS Service Delivery and Organization R&D Programme (SDO/99/2005).
Ethical approval South-East Multi-centre Research Ethics Committee (05/MRE01/109).
Guarantors Hilary Pinnock and Aziz Sheikh are study guarantors.
Contributorship Hilary Pinnock, with the help of Guro Huby and Aziz Sheikh, led the development of the protocol, securing of funding, study administration, data analysis, interpretation of results and writing of the paper. Alison Tierney, Rosemary Porteous and Tara Kielmann undertook data collection and analysis, and led the writing of the paper. All authors reviewed the final manuscript.
Acknowledgments We thank the members of the Independent Steering Committee, Bonnie Sibbald, John Taylor and Donna Covey for their oversight and support of the project. We are also grateful to David Selling for his invaluable advice as the R&D manager for the lead NHS organization, and for his helpful comments on the paper.
References
- 1.Shaw S, Boynton PM, Greenhalgh T. Research governance: where did it come from, what does it mean? J R Soc Med 2005;98: 496-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health. Research Governance Framework for Health and Social Care. 2nd edn. London: DoH, 2005. Available from http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4108962
- 3.Cynulliad Cenedlaethol Cymru. Research Governance Framework for Health and Social Care in Wales. Cardiff: Welsh Assembly, 2001. Available at http://www.word.wales.gov.uk/content/governance/governanceframework-e.pdf
- 4.Primary Care Working Party of the NHS R&D Forum. NHS Information Toolkit for RM&G PCTs. 2nd edition. London: NHS, 2005. Available at http://www.rdforum.nhs.uk/toolkit/toolkit0305.htm
- 5.Primary Care Working Party of the NHS R&D Forum. Information Toolkit for RM&G PCTs. London: NHS, 2003. Available at http://www.rdforum.nhs.uk/toolkit.htm
- 6.Shaw S, Barrett G, Research governance: regulating risk and reducing harm? J R Soc Med 2006;99: 14-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slowther A, Boynton P, Shaw S. Research governance: ethical issues. J R Soc Med 2006;99: 65-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caan W. Research bureaucracy in the United Kingdom: good governance is needed. BMJ 2004;329: 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denhom JT, Research governance is about protection, not convenience. BMJ 2004;329: 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salisbury C, Leese B, McManus R. Ensuring that research governance supports rather than stifles research. Br J Gen Pract 2005;55: 4-5 [PMC free article] [PubMed] [Google Scholar]
- 11.Royal College of General Practitioners. Informal Consultation on Barriers to Research Created by Over-regulation, Ethics Committees. London: Royal College of General Practitioners Research Group, 2004
- 12.Boshier A, Shakir SAW, Telfer P, Behr E, Pakrashi T, Camm AJ. The negative effect of red tape on research. Pharmacoepidemiol Drug Saf 2005;14: 373-6 [DOI] [PubMed] [Google Scholar]
- 13.Leese B, Storey C, Ford J, Cheater F. Researchers' responses to research management and governance for primary care research in England: Persistent and escalating problems over time. J Health Org Manage 2005;19: 494-503 [DOI] [PubMed] [Google Scholar]
- 14.Elwyn G, Seagrove A, Thorne K, Cheung WY. Ethics and research governance in a multicentre study: add 150 days to your study protocol. BMJ 2005;330: 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galbraith N, Hawley C, De-Souza V. Research governance approval is putting people off research. BMJ 2006;332: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne G. A critical account of obtaining research governance approval. Nurse Researcher 2005;13: 2. [DOI] [PubMed] [Google Scholar]
- 17.NHS R&D Forum. Guidance for Researchers on the New NHS R&D Application Form. London: NHS, 2006. Available at http://83.138.142.202/RDFormDocs/RDform_researcher_guidance_pack_200506.pdf
- 18.NHS Service Delivery and Organisation R&D Programme. Workforce SDO Projects. London: NHS, 2005. Available at http://www.sdo.lshtm.ac.uk/sdo992005.html
- 19.Messer J, Boothroyd J. NHS R&D Forum. The NHS R&D Application Form: Frequently Asked Questions. London: NHS, 2005. Available at http://www.rdforum.nhs.uk/docs/rdform_faq_030805.doc
- 20.NHS R&D Forum. The Standard NHS R&D Application Form—A Six Month Review. London: NHS, 2005. Available at http://www.rdforum.nhs.uk/docs/rdform_evaluation_report_1005.doc
- 21.NHS R&D Forum. Developing an Integrated Approval Process for Research in the NHS. Bulletin 2006;2. Available at http://www.rdforum.nhs.uk/docs/Bulletin_Dec06.pdf
- 22.Research and Development Directorate, Department of Health. Best Research for Best Health. A New National Health Research Strategy. London: DoH, 2006. Available at http://www.dh.gov.uk/assetRoot/04/12/71/52/04127152.pdf