Abstract
Objective
Self‐perceived health status may be helpful in identifying patients at high risk for adverse outcomes. The Euro Heart Survey on Coronary Revascularization (EHS‐CR) provided an opportunity to explore whether impaired health status was a predictor of 1‐year mortality in patients with coronary artery disease (CAD) undergoing angiographic procedures.
Methods
Data from the EHS‐CR that included 5619 patients from 31 member countries of the European Society of Cardiology were used. Inclusion criteria for the current study were completion of a self‐report measure of health status, the EuroQol Questionnaire (EQ‐5D) at discharge and information on 1‐year follow‐up, resulting in a study population of 3786 patients.
Results
The 1‐year mortality was 3.2% (n = 120). Survivors reported fewer problems on the five dimensions of the EQ‐5D as compared with non‐survivors. A broad range of potential confounders were adjusted for, which reached a p<0.10 in the unadjusted analyses. In the adjusted analyses, problems with self‐care (OR 3.45; 95% CI 2.14 to 5.59) and a low rating (⩽60) on health status (OR 2.41; 95% CI 1.47 to 3.94) were the most powerful independent predictors of mortality, among the 22 clinical variables included in the analysis. Furthermore, patients who reported no problems on all five dimensions had significantly lower 1‐year mortality rates (OR 0.47; 95% CI 0.28 to 0.81).
Conclusions
This analysis shows that impaired health status is associated with a 2–3‐fold increased risk of all‐cause mortality in patients with CAD, independent of other conventional risk factors. These results highlight the importance of including patients' subjective experience of their own health status in the evaluation strategy to optimise risk stratification and management in clinical practice.
Treatment options for patients with coronary artery disease (CAD) have expanded considerably over the past two decades. In addition to pharmacological therapy, mechanical revascularisation by coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) can be offered to relieve ischaemic symptoms and improve prognosis in some subsets.1,2,3,4,5,6 In addition, behavioural interventions, which include prevention and treatment of lifestyle risk factors and psychological risk factors (eg, anger or anxiety), are known to be beneficial for patients with cardiovascular diseases.7 However, choosing the most appropriate treatment for the individual patient remains controversial in many instances.8
As the observed differences in outcome between competitive treatment options (eg, CABG and PCI) diminish,9,10,11 researchers and clinicians have become increasingly interested in measuring patients' health status. In addition to using health‐related quality of life (HRQL) or health status as an end point in clinical trials, health status may prove useful in the clinical decision‐making process as to which treatment to favour.12,13 It is also important to note that health status is an important patient‐centred outcome, and subsets of patients are known to prefer health status over prolonged survival.14 In addition, measuring health status may help identify patients at high risk for adverse outcomes.12,15,16,17,18 Identification of these patients is important as they may benefit from more invasive management and more intensive follow‐up.17 Yet, health status measures are rarely used in clinical practice.19
The aim of this study was to explore whether impaired health status was a predictor of 1‐year all‐cause mortality in a cohort of patients with established CAD enrolled in the Euro Heart Survey on Coronary Revascularization (EHS‐CR).
Methods
Patients
Data for this study were derived from the database of the EHS‐CR. Details of this prospective, observational study were published previously.20 All consecutive patients undergoing invasive diagnostic or therapeutic procedures in the catheterisation laboratory were screened between November 2001 and March 2002 in 130 hospitals from 31 member countries of the European Society of Cardiology (ESC). Consenting patients with a >50% diameter stenosis in at least one coronary artery were included and detailed information was retrieved from their medical records. The EuroSCORE was calculated from the available variables.21 From the 5619 patients enrolled in the EHS‐CR, 4515 (80%) patients had complete data on all five questions (dimensions) of the EuroQol Questionnaire (EQ‐5D) at baseline. The study protocol included a 1‐year follow‐up, which was available in 3786 (84%) patients.
Health status
In addition to collecting clinical variables, all patients were asked to fill in the self‐report EQ‐5D questionnaire22 at the time of hospital discharge. The EQ‐5D is a standardised generic instrument for assessing health status, with valid translations available for 29 of the 31 participating countries in the current study. This validated questionnaire comprises five dimensions—namely, mobility, self‐care, usual activities, pain or discomfort, and anxiety or depression. Each of these dimensions has three levels of severity, corresponding to “no problems”, “moderate problems” and “severe problems”. Patients were asked which statement best described their health status on the day the questionnaire was filled in. Theoretically, 243 different health states can be generated by this classification. The ratings can be analysed on an individual level using health‐state utility scores. These scores range from –0.594 to 1, with scores <0 being regarded as worse than death and 1 representing full health, from the perspective of the general population.22 The second part of the EQ‐5D consists of a visual analogue scale (VAS) ranging from 0 (best imaginable health state) to 100 (worst imaginable health state), which is used for rating the overall health.
Statistical analysis
Continuous variables are reported as mean or median scores with corresponding values (SD and inter quartiles ranges, respectively). Dichotomous variables are presented as numbers and percentages. To evaluate differences between the different groups, χ2 tests, Student's t test, analysis of variance or Mann–Whitney U tests were applied as appropriate. Univariate and multivariate logistic regression analyses were performed to evaluate the relationship between the five dimensions of the EQ‐5D at baseline and all‐cause mortality at 1 year. To examine the relationship between the dimensions of the EQ‐5D, we dichotomised the three levels of severity: “no problems” was coded 0, whereas “moderate problems” and “severe problems” were coded 1. The VAS was dichotomised by using the lowest 25th centile indicating impaired health. These dichotomised variables were then entered separately in the adjusted analyses. Crude and adjusted odds ratios (ORs) with their corresponding 95% confidence intervals (CIs) are reported. We adjusted for a broad range of potential confounders, which reached a p value of <0.10 in the unadjusted analyses. These variables included age, risk factors, co‐morbidity, admission diagnosis and treatment. Goodness of fit was determined by the Hosmer–Lemeshow test, and discriminatory power was evaluated using c‐statistics. For all tests, a p value <0.05 (two‐sided) was considered significant. Statistical analyses were performed using SPSS V.12.0.1 for Windows.
Results
Table 1 summarises the baseline characteristics of the 3786 patients who were included in the current study, comparing survivors at 1‐year follow‐up with non‐survivors. The all‐cause mortality at 1 year was 3.2% (120 deaths). Cardiac death was observed in 69% of those with a known cause of death (n = 97). Survivors were younger (62.8 vs 69; p<0.001), had a better risk profile (including age, diabetes, cardiovascular history and EuroSCORE), and were more often offered revascularisation (80% vs 63%; p<0.001) as compared with non‐survivors. No significant differences were observed between the admission diagnosis of survivors and non‐survivors. By univariate analysis, conventional variables negatively associated with death were: age, diabetes, peripheral vascular disease, previous myocardial infarction, history of heart failure, previous CABG, multivessel disease, ejection fraction <40%, EuroSCORE and only medical treatment. PCI, use of antiplatelet agents and use of statins were associated with improved outcome. Stable angina was the most frequent indication to perform angiography (54%), followed by non‐ST myocardial infarction or unstable angina (30%) and ST elevation myocardial infarction (15%). On all five EQ‐5D dimensions, survivors reported significantly fewer problems and had a better overall health (ie, VAS) than non‐survivors. The univariate analysis showed that problems on these dimensions were negatively associated with death (table 2). Identical results were observed in a subgroup of patients with cardiac mortality, instead of all‐cause mortality.
Table 1 Baseline characteristics of patients.
| Vital status at 1‐year follow‐up | Univariate predictor for mortality (OR, 95% CI) | p Value | ||
|---|---|---|---|---|
| Alive (n = 3666) | Dead (n = 120) | |||
| Male sex (%) | 2785 (76) | 93 (78) | 1.09 (0.70 to 1.68) | 0.71 |
| Mean (SD) age (years) | 62.8 (10.6) | 69 (9.9) | 1.06 (1.04 to 1.08) | <0.001 |
| Risk factors (%) | ||||
| Smoking ever | 2166 (61) | 75 (63) | 1.05 (0.72 to 1.53) | 0.79 |
| Diabetes mellitus | 850 (23) | 43 (36) | 1.85 (1.26 to 2.70) | 0.002 |
| Hypertension | 2254 (62) | 75 (63) | 1.04 (0.71 to 1.51) | 0.85 |
| Hyperlipidaemia | 2417 (67) | 78 (66) | 0.98 (0.66 to 1.44) | 0.9 |
| Cardiovascular history (%) | ||||
| Peripheral vascular disease | 412 (11) | 32 (27) | 2.87 (1.89 to 4.35) | <0.001 |
| Cerebral vascular disease | 283 (8) | 12 (10) | 1.33 (0.72 to 2.44) | 0.36 |
| Prior myocardial infarction | 1440 (39) | 72 (60) | 2.31 (1.59 to 3.35) | <0.001 |
| Congestive heart failure | 673 (18) | 50 (42) | 3.17 (2.19 to 4.61) | <0.001 |
| Prior percutaneous coronary intervention | 764 (21) | 19 (16) | 0.71 (0.43 to 1.17) | 0.18 |
| Prior coronary artery bypass grafting | 368 (10) | 24 (20) | 2.24 (1.41 to 3.55) | <0.001 |
| Diagnosis at admission (%) | ||||
| Stable angina | 1978 (55) | 60 (52) | 0.85 (0.59 to 1.23) | 0.39 |
| NSTE‐ACS | 1105 (31) | 40 (35) | 1.16 (0.79 to 1.71) | 0.45 |
| STEMI | 537 (15) | 16 (14) | 0.90 (0.53 to 1.53) | 0.90 |
| Angiographic results (%) | ||||
| Multivessel disease | 2308 (63) | 89 (74) | 1.68 (1.11 to 2.54) | 0.01 |
| Left main lesions | 284 (8) | 15 (13) | 1.70 (0.98 to 2.96) | 0.06 |
| Ejection fraction <40% | 296 (12) | 34 (37) | 4.25 (2.74 to 6.60) | <0.001 |
| Mean (SD) EuroSCORE | 4.2 (2.8) | 6.8 (3.4) | 1.28 (1.21 to 1.35) | <0.001 |
| Treatment option (%) | ||||
| Percutaneous coronary intervention | 2201 (60) | 54 (45) | 0.55 (0.38 to 0.79) | 0.001 |
| Coronary artery bypass grafting | 745 (20) | 22 (18) | 0.88 (0.55 to 1.41) | 0.59 |
| Only medical treatment | 720 (20) | 44 (37) | 2.37 (1.62 to 3.46) | <0.001 |
| Medical treatment at discharge (%) | ||||
| Antiplatelet agents/oral anticoagulants | 3464 (95) | 105 (88) | 0.41 (0.23 to 0.71) | 0.002 |
| β‐blockers | 2796 (76) | 86 (72) | 0.79 (0.53 to 1.18) | 0.25 |
| Statins | 2498 (68) | 71 (59) | 0.68 (0.47 to 0.98) | 0.04 |
| ACE inhibitors | 2027 (55) | 76 (63) | 1.40 (0.96 to 2.04) | 0.08 |
NSTE‐ACS, non ST‐elevation acute coronary syndrome; STEMI, ST‐elevation myocardial infarction
Table 2 EuroQol‐5D questionnaire and distribution, before hospital discharge.
| Vital status at 1‐year follow‐up | Univariate predictor for mortality (OR, 95% CI) | p Value | ||
|---|---|---|---|---|
| Alive (n = 3666), n (%) | Dead (n = 120), n (%) | |||
| Mobility | 3 (2.08 to 4.33)* | <0.001 | ||
| I have no problems in walking about | 2579 (70) | 53 (44) | ||
| I have some problems in walking about | 1064 (29) | 61 (51) | ||
| I am confined to bed | 23 (1) | 6 (5) | ||
| Self‐care | 4.64 (3.18 to 6.67)* | <0.001 | ||
| I have no problems with self‐care | 3191 (87) | 71 (59) | ||
| I have some problems washing or dressing myself | 453 (12) | 46 (38) | ||
| I am unable to wash or dress myself | 22 (1) | 3 (3) | ||
| Usual activities (eg, work, housework, family activities) | 3.65 (1.93 to 3.85)* | <0.001 | ||
| I have no problems with performing my usual activities | 2311 (63) | 47 (39) | ||
| I have some problems with performing my usual activities | 1227 (33) | 62 (52) | ||
| I am unable to perform my usual activities | 128 (4) | 11 (9) | ||
| Pain/discomfort | 2.12 (1.47 to 3.05)* | <0.001 | ||
| I have no pain or discomfort | 2295 (63) | 53 (44) | ||
| I have moderate pain or discomfort | 1320 (36) | 59 (49) | ||
| I have extreme pain or discomfort | 51 (1) | 8 (7) | ||
| Anxiety/depression | 2.47 (1.71 to 3.55)* | <0.001 | ||
| I am not anxious or depressed | 2505 (68) | 56 (47) | ||
| I am moderately anxious or depressed | 1061 (29) | 54 (45) | ||
| I am extremely anxious or depressed | 100 (3) | 10 (8) | ||
| EQ‐VAS | 3.45 (2.29 to 5.19)† | <0.001 | ||
| Mean (SD) | 69 (19) | 57 (23) | ||
| Median, interquartile range | 70 (60 to 80) | 58 (45 to 80) | ||
| EQ‐utility score | 2.70 (1.87 to 3.9)† | <0.001 | ||
| Mean (SD) | 0.81 (0.23) | 0.63 (0.34) | ||
| Median, interquartile range | 0.85 (0.69 to 1.0) | 0.71 (0.52 to 0.85) | ||
EQ, EuroQol; EQVAS, EuroQol visual analogue scale; VAS, visual analogue scale.
*Patients indicating problems on the EQ‐5D dimension.
†Dichotomised (using the lowest 25th centile indicating impaired health status).
Table 3 shows the adjusted association between the EQ‐5D and 1‐year mortality. Patients who reported problems on perceived health status and patients who had a relatively low score (⩽60) on the EQ‐VAS had a higher mortality rate as compared with patients who reported no problems. Problems with self‐care (OR 3.45; 95% CI 2.14 to 5.59) and a health rating ⩽60 (OR 2.41; 95% CI 1.47 to 3.94) were the most powerful predictors of mortality. Furthermore, patients who reported no problems on all five dimensions had significantly lower 1‐year mortality (OR 0.47; 95% CI 0.28 to 0.81), whereas patients who reported problems on all dimensions were in the highest risk group (OR 3.85; 95% CI 2.30 to 6.44). The EQ‐5D improved the model c‐statistics (from 0.78 to 0.81). Calibration was good for the adjusted analyses as Hosmer–Lemeshow tests showed no significant difference between the observed and predicted probabilities.
Table 3 Adjusted association between the dimensions of the EuroQol‐5D Questionnaire and all‐cause mortality*.
| Mobility‡ | Self‐care‡ | Usual activities‡ | Pain/discomfort‡ | Anxiety/depression‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Clinical variables† | ||||||||||
| Age (years) | 1.03 (1 to 1.06) | 0.09 | 1.03 (1 to 1.06) | 0.08 | 1.03 (1 to 1.06) | 0.07 | 1.03 (1 to 1.06) | 0.05 | 1.03 (1 to 1.07) | 0.05 |
| History of HF, n | 1.63 (0.99 to 2.67) | 0.06 | 1.52 (0.92 to 2.51) | 0.10 | 1.62 (0.98 to 2.66) | 0.06 | 1.65 (1 to 2.71) | 0.05 | 1.67 (1.01 to 2.75) | 0.04 |
| EF <40% | 1.87 (1.1 to 3.20) | 0.02 | 1.82 (1.06 to 3.13) | 0.03 | 1.88 (1.1 to 3.21) | 0.02 | 1.83 (1.07 to 3.13) | 0.03 | 1.88 (1.1 to 3.22) | 0.02 |
| Prior MI, n | 1.96 (1.22 to 3.14) | 0.006 | 1.9 (1.18 to 3.05) | 0.008 | 1.93 (1.2 to 3.1) | 0.007 | 1.96 (1.22 to 3.16) | 0.005 | 1.95 (1.21 to 3.14) | 0.006 |
| PVD | 1.98 (1.1 to 3.56) | 0.02 | 2.19 (1.21 to 3.99) | 0.01 | 2.01 (1.11 to 3.62) | 0.02 | 1.91 (1.06 to 3.45) | 0.03 | 2.02 (1.12 to 3.64) | 0.02 |
| Medical treatment | 2.07 (1.05 to 4.07) | 0.04 | 2.26 (1.15 to 4.47) | 0.02 | 2.12 (1.08 to 4.16) | 0.03 | 2.13 (1.09 to 4.18) | 0.03 | 2.06 (1.05 to 4.05) | 0.04 |
| EQ‐5D dimensions‡ | 2.2 (1.39 to 3.5) | <0.001 | 3.45 (2.14 to 5.59) | <0.001 | 2.13 (1.34 to 3.38) | <0.001 | 2.12 (1.33 to 3.37) | 0.001 | 2.31 (1.48 to 3.59) | <0.001 |
EF, ejection fraction; EQ, EuroQol; HF, heart failure; MI, myocardial infarction; PVD, peripheral vascular disease; VAS, visual analogue scale.
*Adjusted for age, diabetes, peripheral vascular disease, previous myocardial infarction, history of heart failure, previous coronary artery bypass grafting, multivessel disease, left main, ejection fraction <40%, EuroSCORE, percutaneous coronary intervention, medical treatment, anti‐platelet agents, statins and ACE‐inhibitors, which reach a p value of 0.10 in the unadjusted analyses (table 1).
†Only clinical variables that remained statistically significant in the adjusted analyses are described.
‡The five dimensions were entered separately in the adjusted analyses.
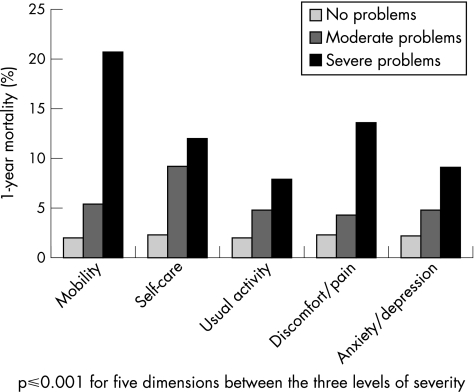
Figure 1 shows per dimension that patients who reported no problems had a low mortality (<3%), whereas patients who had moderate or severe problems had considerably higher mortality (range 4–21%).
Figure 1 One‐year mortality stratified by the EuroQol‐5D dimensions.
As 33% of all patients enrolled in the EHS‐CR were excluded from this analysis, we compared the baseline characteristics of these patients with the study population. With the exception of a higher in‐hospital mortality in those who were excluded (5.1% vs 0.3%), no major differences were observed.
Discussion
This study showed that impaired health status, as measured by the EQ‐5D before discharge, is associated with a 2–3‐fold increased risk of all‐cause mortality in patients with established CAD. After adjustment for other prognostic variables, including age, risk factors, comorbidity and admission diagnosis, impaired health status remained an independent predictor of 1‐year mortality.
Several studies have reported on the predictive value of HRQL and health status questionnaires in relation to adverse clinical outcomes in patients with cardiovascular diseases.15,16,18,23 To our knowledge, this study is the first to use the EQ‐5D, a brief generic self‐perceived health status questionnaire, to predict short‐term mortality (ie, after 1 year) independently of established biomedical risk factors in patients with CAD with a relatively low overall risk. We identified reduced self‐care as the most powerful predictor of mortality. Of note, this dimension is strongly related to patients' abilities to care for themselves and adequately manage their condition. As a consequence, targeting and improving self‐care behaviour in intervention programmes could not only lead to improved HRQL but also enhance survival in this subset of patients.24,25 In addition, a major advantage of the EQ‐5D is that it is a brief and valid measure of health status that can be easily used in clinical practice.
Our findings support the recommendations of Krumholz et al17 to include health status measurements in clinical practice as an additional tool to identify patients who are at high risk for adverse outcomes. These patients may consequently benefit from a more aggressive treatment, including invasive, pharmacological and/or behavioural interventions or a combination hereof. An earlier report on the EHS‐CR showed that there is adequate room for improvement in the medical treatment of these patients, especially with respect to adjunctive pharmacology (glycoprotein IIb/IIIa inhibitors, statins and ACE inhibitors).20 Another important issue for advocating the use of health status assessment in clinical practice relates to the issue of discrepancy between patient‐rated and physician‐rated health status.26 As clinicians frequently underestimate patients' health status as reported by their patients,27 it is paramount that patients' evaluation of “how they feel” is taken into account. In addition, health status is an important patient‐centred outcome, with patients emphasising health status over prolonged survival.14 Hence, entering health status into the equation when discussing treatment options with patients may also be considered an ethical obligation.
Although this study clearly showed that the EQ‐5D provides prognostic information, little is known about the “how and why” impaired health status predicts mortality, independently of biomedical risk factors. It should be noted, however, that health status involves a much broader range of the effect of disease as experienced by the patient (ie, symptoms, functional limitation, and discrepancy between actual and desired function) compared with the focus of clinicians (ie, symptoms, signs and diagnosis).19 Further research is warranted into the mechanisms that may be responsible for the relationship between health status and mortality, as this could guide treatment with or the development of effective interventions. Emphasis should also be placed on the identification of the determinants of impaired health status, which has been advocated as a means to close the gap between research and clinical practice.17 Both depression and the distressed (type D) personality have been shown to predict impaired health status adjusting for measures of severity of disease and other risk factors.28,29 The question is whether these psychosocial risk factors are more important determinants of individual differences in clinical outcome than health status.
This study is the first to use the EQ‐5D as a predictor of mortality. Although other generic and disease‐specific health status questionnaires have been found to predict mortality, one of the major advantages of the EQ‐5D is its brevity. It comprises only six questions, whereas most of the other questionnaires ask many more questions (range 19–36) and are more taxing to patients.12,15,16,23 In addition, it is important to note that in patients with CAD a simple questionnaire such as EQ‐5D is able to discriminate between patients who have a higher mortality risk and those who do not. By contrast, we acknowledge that a lack of familiarity with the concept of health status, the perception of many clinicians that health status is a soft end point in evaluating a treatment19 and the high workload of physicians in clinical practice may be identified as barriers for implementing self‐perceived health status in every day clinical practice. However, it should be noted that it takes less than 5 min to complete the questionnaire, and health care professionals other than physicians can become involved in the assessment.
The current study has several potential limitations. Firstly, patients who did not complete the EQ‐5D questionnaire or who had missing follow‐up data had to be excluded from analyses. However, a comparison between responders and non‐responders did not show major differences. Secondly, it cannot be excluded that ill health conditions, other than cardiovascular diseases, could have had an effect on the results, as only “classical” risk factors and comorbidities were included in the database. Thirdly, health status was assessed only once, and at that time not all patients had undergone a revascularisation procedure. Finally, we used a generic rather than a disease‐specific instrument to evaluate health status; it is well known that generic measures may be less sensitive than disease‐specific measures to tap dimensions pertinent to clinical populations. Future research is needed to consider issues such as the predictive value of a single measurement as compared with serial measurements, the effect of changes in health status over time on outcomes, and comparing the results of the EQ‐5D with disease‐specific instruments. Despite these limitations, strengths of this study were the relatively large number of patients included from multiple hospitals across Europe. We were also able to adjust for a number of classical demographic and cardiovascular risk factors, showing that impaired health status is an independent predictor for mortality. Lastly, the enrolled patients are representative of “real life” practice, across a wide spectrum of European hospitals.
In conclusion, this study showed the strong incremental value of the EQ‐5D for the prediction of mortality in patients admitted with CAD, independently of other demographic, clinical and angiographic risk factors. Our results highlight the importance of including patients' subjective experience of their own health status to optimise risk stratification in clinical practice.
Acknowledgements
We thank the Euro Heart Survey Team, national coordinators, participating centres, local investigators and data‐collecting officers. The Euro Heart Survey was supported by industry sponsors and supporting institutions as published earlier. MJ Lenzen was supported by the Netherlands Heart Foundation (2000T101).
Abbreviations
CABG - coronary artery bypass grafting
CAD - coronary artery disease
EHS‐CR - Euro Heart Survey on Coronary Revascularization
EQ‐5D - EuroQol‐5D
HRQL - health‐related quality of life
PCI - percutaneous coronary intervention
VAS - visual analogue scale
Footnotes
Competing interest: None.
References
- 1.Julian D, Bertrand M, Hjalmarsson A.et al Management of stable angina pectoris. Recommendations of the Task Force of the European Society of Cardiology. Eur Heart J 199718394–413. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand M E, Simoons M L, Fox K A.et al Task Force on the management of acute coronary syndromes of the European Society of Cardiology. Management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J 2002231809–1840. [DOI] [PubMed] [Google Scholar]
- 3.Van de Werf F, Ardissino D, Betriu A.et al Management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J 20032428–66. [DOI] [PubMed] [Google Scholar]
- 4.Smith S C, Jr, Dove J T, Jacobs A K.et al ACC/AHA Guidelines for percutaneous coronary intervention (Revision of the 1993 PTCA Guidelines)—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation 20011033019–3041. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons R J, Abrams J, Chatterjee K.et al ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation 2003107149–158. [DOI] [PubMed] [Google Scholar]
- 6.Eagle K A, Guyton R A, Davidoff R.et al ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1999 guidelines for coronary artery bypass graft surgery). Circulation 20041101168–1176. [DOI] [PubMed] [Google Scholar]
- 7.Pickering T, Clemow L, Davidson K.et al Behavioral cardiology—has its time finally arrived? Mt Sinai J Med 200370101–112. [PubMed] [Google Scholar]
- 8.Mercado N, Wijns W, Serruys P W.et al One‐year outcomes of coronary artery bypass graft surgery versus percutaneous coronary intervention with multiple stenting for multisystem disease: a meta‐analysis of individual patient data from randomized clinical trials. J Thorac Cardiovasc Surg 2005130512–519. [DOI] [PubMed] [Google Scholar]
- 9.Pocock S J, Henderson R A, Rickards A F.et al Meta‐analysis of randomised trials comparing coronary angioplasty with bypass surgery. Lancet 19953461184–1189. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman S N, TenBrook J, John A.et al A meta‐analysis of randomized controlled trials comparing coronary artery bypass graft with percutaneous transluminal coronary angioplasty: one‐ to eight‐year outcomes. J Am Coll Cardiol 2003411293–1304. [DOI] [PubMed] [Google Scholar]
- 11.Feit F, Brooks M M, Sopko G.et al Long‐term clinical outcome in the bypass angioplasty revascularization investigation registry: comparison with the randomized trial. Circulation 20001012795–2802. [DOI] [PubMed] [Google Scholar]
- 12.Spertus J A, Jones P, McDonell M.et al Health status predicts long‐term outcome in outpatients with coronary disease. Circulation 200210643–49. [DOI] [PubMed] [Google Scholar]
- 13.Borkon A M, Muehlebach G F, House J.et al A comparison of the recovery of health status after percutaneous coronary intervention and coronary artery bypass. Ann Thorac Surg 2002741526–1530. [DOI] [PubMed] [Google Scholar]
- 14.Stanek E J, Oates M B, McGhan W F.et al Preferences for treatment outcomes in patients with heart failure: symptoms versus survival. J Card Fail 20006225–232. [DOI] [PubMed] [Google Scholar]
- 15.Soto G E, Jones P, Weintraub W S.et al Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004110546–551. [DOI] [PubMed] [Google Scholar]
- 16.Rumsfeld J S, MaWhinney S, McCarthy M., Jret al Health‐related quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the Department of Veterans Affairs Cooperative Study Group on processes, structures, and outcomes of care in cardiac surgery. JAMA 19992811298–1303. [DOI] [PubMed] [Google Scholar]
- 17.Krumholz H M, Peterson E D, Ayanian J Z.et al Report of the National Heart, Lung, and Blood Institute working group on outcomes research in cardiovascular disease. Circulation 20051113158–3166. [DOI] [PubMed] [Google Scholar]
- 18.Heidenreich P A, Spertus J A, Jones P G.et al Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol 200647752–756. [DOI] [PubMed] [Google Scholar]
- 19.Rumsfeld J S. Health status and clinical practice: when will they meet? Circulation 20021065–7. [DOI] [PubMed] [Google Scholar]
- 20.Lenzen M J, Boersma E, Bertrand M E.et al Management and outcome of patients with established coronary artery disease: the Euro Heart Survey on coronary revascularization. Eur Heart J 2005261169–1179. [DOI] [PubMed] [Google Scholar]
- 21.Nashef S A, Roques F, Michel P.et al European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999169–13. [DOI] [PubMed] [Google Scholar]
- 22.Dolan P D. Modeling valuations for EuroQol health states. Med Care 1997351095–1108. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez‐Artalejo F, Guallar‐Castillon P, Pascual C R.et al Health‐related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med 20051651274–1279. [DOI] [PubMed] [Google Scholar]
- 24.Grady K L, Dracup K, Kennedy G.et al Team management of patients with heart failure: a statement for healthcare professionals from The Cardiovascular Nursing Council of the American Heart Association. Circulation 20001022443–2456. [DOI] [PubMed] [Google Scholar]
- 25.Jaarsma T, Stromberg A, Martensson J.et al Development and testing of the European Heart Failure Self‐Care Behaviour Scale. Eur J Heart Fail 20035363–370. [DOI] [PubMed] [Google Scholar]
- 26.Calkins DR R L, Cleary P D, Davies A R.et al Failure of physicians to recognize functional disability in ambulatory patients. Ann Intern Med 1991114451–454. [DOI] [PubMed] [Google Scholar]
- 27.Kind P, Dolan P, Gudex C.et al Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998316736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rumsfeld J S, Ho P M. Depression and cardiovascular disease: a call for recognition. Circulation 2005111250–253. [DOI] [PubMed] [Google Scholar]
- 29.Schiffer A A, Pedersen S S, Widdershoven J W.et al The distressed (type D) personality is independently associated with impaired health status and increased depressive symptoms in chronic heart failure. Eur J Cardiovasc Prev Rehabil 200512341–346. [DOI] [PubMed] [Google Scholar]