Abstract
Objective
To investigate the association between chronic exposure to second hand smoke (SHS) and the short‐term prognosis of patients hospitalised with acute coronary syndromes.
Methods
Between 1 October 2003 and 30 September 2004, 2172 consecutive patients enrolled with acute coronary syndromes at the cardiology clinics or the emergency units of six major hospitals, in Greece were studied. Exposure to SHS was measured through a questionnaire administered during a specific interview, after the second day of hospitalisation. The main outcome of interest was the 30‐day status of these patients (death, or rehospitalisation due to coronary heart disease).
Results
1003 (46%) of the patients were exposed to SHS. Patients reporting exposure to SHS had 61% (95% CI 14% to 118%) higher risk of having an event during the first 30 days after hospitalisation as compared with patients who were not exposed to SHS, after taking into account the effect of several potential confounders. A dose–response linear relationship was observed between the risk of having recurrent events and the years of exposure to SHS (ρ = 0.17, p<0.001).
Conclusions
Exposure to SHS increases considerably the risk of recurrent events in patients who had survived a cardiac event.
Breathing second hand smoke (SHS) or “passive smoking” has been investigated in several studies.1,2,3,4 It is believed that the risk of illness from SHS is 2%–10% that of the risk from active smoking.1 Nevertheless, the effect of environmental exposure to tobacco smoke on human health has not been well understood and appreciated, and reports have documented that several investigators are trying to mislead the public.5 In the context of the Greek study of acute coronary syndromes epidemiological study that was designed to evaluate clinical, lifestyle and behavioural factors associated with the severity and prognosis of patients hospitalised with acute coronary syndromes (ACS),6 we tested the hypothesis whether chronic exposure to SHS influences the 30‐day prognosis of these patients.
Methods
Study sample
Between 1 October 2003 and 30 September 2004, we enrolled 2172 patients admitted for ACS in six major general hospitals in Greece (Hippokration Hospital in Athens, and the general hospitals in Lamia, Karditsa, Halkida, Kalamata and Zakynthos Island). Of them, 1649 were men, 65 (13) years old and 523 were women, 72 (11) years old. The enrolled participants were almost all patients, who were hospitalised in the aforementioned hospitals during this period (the participation rate varied from centre to centre from 85% to 99%). At entry a 12‐lead ECG was performed and clinical symptoms were evaluated in all patients. On the basis of the ECG findings, patients were classified as having ST‐segment elevations, non‐ST segment elevations or other ECG findings. Moreover, blood tests were performed to detect evidence of myocardial cell death. We measured troponin I levels and the MB fraction of total creatinine phosphokinase (CPK). We included only cases with discharge diagnoses of acute coronary syndromes (acute myocardial infarction or unstable angina). In particular, acute myocardial infarction was defined by at least two of the following features: (a) ECG changes (patients with or without ST segment elevations), (b) compatible clinical symptoms and (c) specific diagnostic sensitive biomarker—elevations (troponin I >0.4 ng/ml and the MB fraction of CPK >8.8 ng/ml).7 Unstable angina was defined by the occurrence of one or more angina episodes, at rest, within the preceding 48 h, corresponding to class III of the Braunwald classification.8 According to the discharge diagnosis, 764 (35%) patients were diagnosed as having unstable angina, 699 (32%) patients as having non‐Q‐wave myocardial infarction and 709 (33%) patients as having Q‐wave myocardial infarction.
Exposures to SHS
With the exception of 15 patients who died during the first 24 h of their admission, a detailed medical history was recorded from all other patients using accurate medical records. Exposure to SHS was measured through a confidential questionnaire administered during a specific interview by the study's investigators, after the second day of hospitalisation. In particular, the questionnaire asked “Are you currently exposed to tobacco smoke from other people for more than 30 min per day?” If the answer was “yes”, we also asked for the duration and the location (ie, home and workplace) of exposure. In addition to the question on current exposure to environmental tobacco smoke, we also asked “As an adult, how many years have you lived with someone who has smoked regularly?” As these were self‐reported assessments and prone to bias, we compared these results to reports obtained from subjects' relatives or friends. The Kendal's τ coefficient showed high concordance between the answers of the investigated patients and their relatives or friends (available information in 76% of the patients) regarding the years of exposure to SHS (τ = 0.75, p = 0.01).
In this work, the main outcome of interest was the 30‐day status of patients who survived from an acute coronary syndrome (death, or rehospitalisation due to coronary heart disease).
Other measurements and characteristics
Following established criteria we recorded previous hospitalisation for cardiovascular disease (ie, coronary heart disease, stroke or other cardiovascular disease), presence and management of hypertension, hypercholesterolaemia, renal failure and diabetes mellitus. Height and weight was measured, to the nearest 0.5 cm and 100 g, respectively. Body mass index (BMI) was then calculated as weight (in kg) divided by height (in m2). Overweight was defined as BMI between 25 and 29.9 kg/m2, whereas obesity as BMI >29.9 kg/m2. Sociodemographic characteristics included age, sex and years of education. Current smokers were defined as those who smoked at least one cigarette per day or had stopped cigarette smoking during the past 12 months. Former smokers were defined as those who had stopped smoking >1 year previously. The rest were defined as never smokers or rare smokers. To evaluate physical activity status of the patients during the past year, we used a modified version of a self‐reported questionnaire. On the basis of this questionnaire, we assessed the frequency (times per week), duration (in minutes) and intensity of sports or occupation‐related physical activity. Participants who did not report any physical activities were defined as sedentary. The evaluation of the nutritional habits was based on a semiquantitative food frequency questionnaire. The consumption of certain food items (non‐refined cereals and products, fruits and nuts, vegetables, olive oil, non‐fat or low‐fat dairy, fish, poultry, potatoes, pulses, eggs, sweets, red meat and meat products, wine, fat and high monounsaturated‐to‐saturated fat ratio) and the portion size as an average per week, during the past year, was recorded. Then, the frequency of consumption was quantified approximately in terms of the number of times a month the food was consumed and a diet score has been developed that indicates adherence to a healthy dietary pattern (range 0–55). Further details about the aims and procedures of the Greek study of acute coronary syndromes may be found elsewhere.6
The study was approved by the Medical Research Ethics Committee of our institution and was carried out in accordance with the Declaration of Helsinki (1989) of the World Medical Association.
Statistical analysis
Continuous variables are presented as mean (SD), whereas categorical variables are presented as absolute and relative frequencies. The non‐parametric Spearman ρ correlation coefficient evaluated relationships between continuous variables because of the skewed distributions of the measurements. Contingency tables with calculation of χ2 test assessed the associations between categorical variables. Mann–Whitney U and Kruskal–Wallis tests evaluated the associations between the groups of patients and continuous variables, as the latter were not normally distributed. The estimates of the risk ratios (RRs) of recurrent events during the first 30 days after hospitalisation were performed through multiple logistic regression analysis, after adjusting for age, sex, clinical characteristics and lifestyle habits. Deviance residuals were calculated to evaluate the estimated model's goodness‐of‐fit. The exposed attributable fraction was calculated by the formula: exposed attributable fraction = (RR–1)/risk ratio. We calculated the absolute risk of having a recurrent event as a function of age, sex, history of coronary heart disease and discharge diagnosis. All reported probability values (p values) were based on two‐sided tests and compared with a statistically significant level of 5%. STATA V.6 software was used for the all the calculations.
Results
Of the 2172 patients with ACS, 42% of women and 33% of men had unstable angina, 34% of women and 32% of men had non‐Q‐wave myocardial infarction and 24% of women versus 35% of men had Q‐wave myocardial infarction. During the first 30 days after hospitalisation, precise information about vital status or rehospitalisation because of coronary heart disease problems was retrieved from 1683 of 2172 patients. The number of events was 119 in men (9.2%) and 38 in women (9%), whereas 41 of them were fatal (25 in men, p for sex differences = 0.003). Table 1 shows the distribution of several characteristics of the patients stratified by the outcome at 30‐day after hospitalisation.
Table 1 Characteristics of the patients with acute coronary syndromes according to the 30‐day outcome.
| Event‐free (n = 2015) | Death or rehospitalisation (n = 157) | p Value | |
|---|---|---|---|
| Sociodemographic and lifestyle characteristics | |||
| Age, years | 66 (13) | 66 (12) | 0.93 |
| Male sex, n (%) | 1511 (75) | 119 (76) | 0.75 |
| Years of school | 7 (4) | 8 (4) | 0.87 |
| Sedentary, n (%) | 1209 (60) | 119 (76) | 0.12 |
| Diet score (0–55) | 28 (5) | 26 (4) | 0.03 |
| Ever smokers, n (%) | 1350 (67) | 107 (68) | 0.74 |
| Current smokers, n (%) | 625 (31) | 57 (36) | 0.09 |
| Exposure to SHS, n (%) | 967 (48) | 97 (62) | <0.001 |
| Exposure to SHS at work, n (%) | 846 (42) | 83 (53) | <0.001 |
| Exposure to SHS at home, n (%) | 443 (22) | 39 (25) | 0.18 |
| Discharge diagnosis | |||
| Unstable angina, n (%) | 544 (27) | 61 (39) | <0.001 |
| Non‐Q‐wave MI, n (%) | 786 (39) | 46 (29) | 0.001 |
| Q‐wave MI, n (%) | 685 (34) | 50 (32) | 0.42 |
| Clinical characteristics | |||
| Previous CHD, n (%) | 806 (40) | 60 (38) | 0.46 |
| Family history of CHD, n (%) | 625 (31) | 53 (34) | 0.25 |
| Obesity (BMI> 29.9 kg/m2), n (%) | 463 (23) | 30 (19) | 0.17 |
| Hypertension, n (%) | 967 (48) | 82 (52) | 0.42 |
| Hypercholesterolaemia, n (%) | 866 (43) | 77 (49) | 0.05 |
| Diabetes mellitus, n (%) | 685 (34) | 47 (30) | 0.16 |
BMI, body mass index; CHD, coronary heart disease; MI, myocardial infraction; SHS, second hand smoke.
The analysis of the data showed that 1003 (46%) patients reported that they were exposed to SHS, independently from their smoking status (current, former or never smokers); 35% of those who reported exposure to SHS were non‐smokers. Moreover, 39% of patients reported that they were exposed at work, 21% at home, and the remainder at other places. A positive correlation was observed between years of exposure and maximum troponin I, CPK levels (ρ = 0.23, p = 0.001 and ρ = 0.13, p = 0.001, respectively). Furthermore, patients with Q‐wave myocardial infarction reported longer exposure to SHS compared with those who had non‐Q‐wave myocardial infarction or unstable angina (32 (16) vs 29 (16) vs 28 (17), p = 0.009).
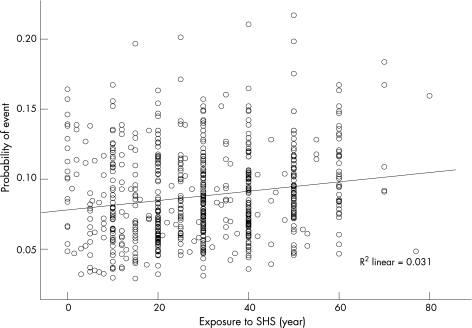
In all, 11% of patients who were exposed to SHS and 8% of patients who were not exposed had a recurrent event during the 30‐day follow‐up (unadjusted risk ratio (RR) 1.42, 95% confidence interval (CI) 1.02 to 1.97, p = 0.03). Moreover, patients who had had an event during the 30‐day follow‐up period reported longer duration of exposure to someone else's smoke compared with those who had not had an event (11 (16) vs 8 (14) years, p = 0.002). We also observed higher risk ratios of recurrent events because of exposure to SHS in patients who currently smoke (RR 2.67, 95% CI 1.83 to 3.89) compared with non‐smokers (RR 1.37, 95% CI 1.11 to 1.64). We also calculated the absolute risk of having a recurrent event as a function of age, sex, history of coronary heart disease and discharge diagnosis. A dose–response linear relationship was observed between the probability of having recurrent events and the years of exposure to SHS (fig 1, r = 0.14, p<0.001).
Figure 1 Probability of a recurrent event by years of exposure to SHS.
However, residual confounding may exist, thus we adjusted for the effect of age, sex, discharge diagnosis, presence of obesity, clinical history of hypertension, hypercholesterolaemia, diabetes, coronary heart disease, family history of coronary heart disease, as well as smoking and physical activity status. Multivariate analysis showed that patients reporting exposure to SHS had 61% higher risk of having an event during the first 30 days after the event as compared with patients who were not exposed to SHS (table 2). When we focused only on non‐smokers, we found that SHS at work was significantly associated with higher risk of recurrent events (RR 1.73, 95% CI 1.01 to 3.2). We attempted to refine the exposure categories further by examining the separate effects of exposure at workplace or at home. We observed that exposure at workplace increased 2.1‐fold (95% CI 1.56 to 2.98) the risk of an event during short term follow up, after controlling for the aforementioned confounders, whereas no association was observed between exposure to SHS at other places.
Table 2 Results from the multiple logistic regression analysis that evaluated the association of second hand smoke exposure on the 30‐day prognosis of patients with acute coronary syndromes.
| RR | 95% CI | |
|---|---|---|
| Exposure vs no exposure to SHS | 1.61 | 1.14 to 2.28 |
| Age (per year) | 1.04 | 1 to 1.08 |
| Male vs female sex | 1.27 | 0.59 to 2.68 |
| Discharge diagnosis (MI vs UA) | 1.85 | 1.25 to 2.74 |
| Current smoking (yes/no) | 1.25 | 0.76 to 2.08 |
| Hypertension (yes/no) | 0.97 | 0.43 to 2.95 |
| Hypercholesterolaemia (yes/no) | 1.47 | 0.71 to 3 |
| Diabetes mellitus (yes/no) | 2.54 | 1.22 to 5.25 |
| Family history of CHD (yes/no) | 1.37 | 0.64 to 2.35 |
| Previous CHD (yes/no) | 0.47 | 0.22 to 0.97 |
| Obesity (yes/no) | 1.01 | 0.43 to 2.98 |
| Physical activity (yes/no) | 0.48 | 0.2 to 1.12 |
| Education (per 1‐year of school) | 1.02 | 0.92 to 1.14 |
| Diet score (per 5 units) | 0.8 | 0.6 to 0.97 |
CHD, coronary heart disease; MI, myocardial infraction; UA, unstable angina; SHS, second hand smoke.
No significant differences were observed when we split the analysis according to the discharge diagnosis (ie, myocardial infarction or unstable angina). Finally, no significant interactions were observed between the educational level and exposure to environmental tobacco smoke when the other covariates were taken into account (p = 0.11). According to these findings, and from a public health perspective, we could say that 45% of the events among those exposed to SHS are attributable to SHS (exposed attributed risk).
Discussion
On the basis of a large sample of hospitalised patients for an acute coronary event, we observed that those who were passively exposed to tobacco smoke, especially at the workplace, experience a greater risk of recurrent events within 30 days after hospitalisation, compared with those who are not exposed to environmental smoke. This is one of the first studies that assessed the effect of chronic exposure to SHS on the short‐term prognosis of patients hospitalised for ACS.
During the past years special attention has been given to the effect of exposure to SHS on human health, especially to the incidence of lung cancer and cardiovascular disease. Reports from several epidemiological studies suggest that SHS produces more arteriosclerosis9,10,11 and increases the risk of coronary heart disease by 25% for a non‐smoker compared with that of unexposed individuals.12 This is mainly caused by changes in cholesterol levels, aggregation of platelets, damage of endothelial cells in the arteries and increased inflammatory markers levels.4,9,10,11 In a recent review paper the authors suggested that the relatively low doses of toxins inhaled by exposure to SHS are sufficient to elicit acute endothelial dysfunction, and at least in part, to the inactivation of nitric oxide.13 Additionally, SHS induces oxidative stress and promotes vascular inflammation.13 Robinson et al14 showed that the creatinine kinase levels of active smokers experiencing ACS were higher than that of non‐smokers. Our findings add to the current scientific knowledge by showing that the exposure to SHS substantially increases the risk of short‐term recurrent events in hospitalised patients for ACS. Also, it seems that the increased risk among never smokers is roughly half the increase among active smokers. Taking into account that the risk of recurrent events in people who have had a cardiac event is much higher during the first 30 days after the event, chronic exposure to SHS seems to add significantly to the excess risk.
Although people's right not to be exposed to other people's tobacco smoke at the workplace has become increasingly recognised over the past years, it seems that in Greece (and probably in other regions) this right has been clearly infringed. Since the late 1980s several ministerial decisions (A2/1989, A2g/1980, 4508/1990 and Y3/4322/1993) have prohibited smoking in hospitals, private clinics, in places belonging to state agencies, in private or public companies and organisations, as well as during the flights of all domestic airlines. Nevertheless, 45% of the recurrent events in our sample were attributable to the exposure to SHS especially at workplace. The latter may be due to the greater duration and intention of exposure.
Conclusion
Despite the limitations that our study might have (ie, self reports of exposure, lack of biochemical evaluation of exposure, information about continuing smoking or exposure to SHS after discharge), it can be stated clearly that exposure to SHS increases considerably the risk of recurrent coronary events among people with ACS.
Acknowledgements
We thank the field investigators of the Greek study of acute coronary syndromes study: Yannis Kogias, Yannis Mantas, Spyros Zombolos, Antonis Antonoulas, Petros Stravopodis, Sophia Arapi, George Giannopoulos, Theodoros Gialernios, Constandina Massoura, George Papanagnou, Antonis Karanasios, Lambros Rizos, Michalis Mparmparoussis, George Kassimatis, Skevos Sideris and Nick Daskalopoulos, for their support in the clinical evaluation and Alexander Chalamandaris for the database management.
Abbreviations
ACS - acute coronary syndromes
BMI - body mass index
CPK - creatinine phosphokinase
SHS - second hand smoke
Footnotes
Competing interests: None declared.
References
- 1.Barnoya J, Glantz S A. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 20051112684–2698. [DOI] [PubMed] [Google Scholar]
- 2.Kawachi I. More evidence on the risks of passive smoking. BMJ 2005330265–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He J, Vupputuri S, Allen K.et al Passive smoking and the risk of coronary heart disease: a meta‐analysis of epidemiologic studies. N Engl J Med 1999340920–992. [DOI] [PubMed] [Google Scholar]
- 4.Panagiotakos D B, Pitsavos C. Passive smoking's role in diabetes. BMJ 20063321044–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diethelm P A, Rielle J C, McKee M. The whole truth and nothing but the truth? The research that Philip Morris did not want you to see. Lancet 200536686–92. [DOI] [PubMed] [Google Scholar]
- 6.Pitsavos C, Panagiotakos D B, Antonoulas A.et al Epidemiology of acute coronary syndromes in a Mediterranean country; aims, design and baseline characteristics of the Greek study of acute coronary syndromes (GREECS). BMC Public Health 2005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Society of Cardiology, American College of Cardiology Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 200021502–1513. [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E.Heart disease. 5th edn. WB Saunders Company, London, UK 1997
- 9.Roberts K A, Rezai A A, Pinkerton K E.et al Effect of environmental tobacco smoke on LDL accumulation in the artery wall. Circulation 1996942248–2253. [DOI] [PubMed] [Google Scholar]
- 10.Davies J, Shelton L, Watanabe I.et al Passive smoking affects endothelium and platelets. Arch Intern Med 19891068–1072. [PubMed]
- 11.Ciruzzi M, Pramparo P, Esteban O.et al Case‐control study of passive smoking at home and risk of acute myocardial infarction. J Am Coll Cardiol 199831797–803. [DOI] [PubMed] [Google Scholar]
- 12.Garland C, Barrett‐Connor E, Suarez L.et al Effects of passive smoking on ischaemic heart disease mortality of nonsmokers: a prospective study. Am J Epidemiol 1985121645–650. [DOI] [PubMed] [Google Scholar]
- 13.Raupach T, Schafer K, Konstantinides S.et al Secondhand smoke as an acute threat for the cardiovascular system: a change in paradigm. Eur Heart J 200627386–392. [DOI] [PubMed] [Google Scholar]
- 14.Robinson K, Conroy R M, Mulcahy R. Smoking and acute coronary heart disease: a comparative study. Br Heart J 198860465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]