Abstract
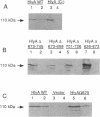
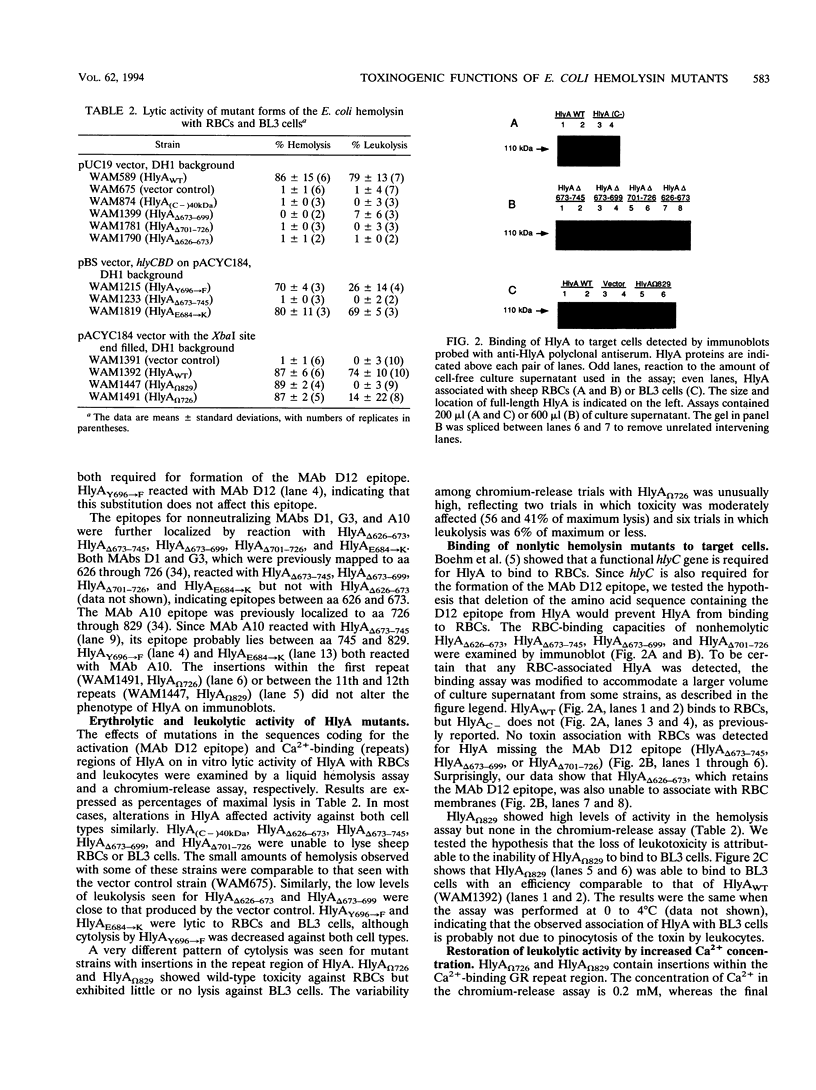
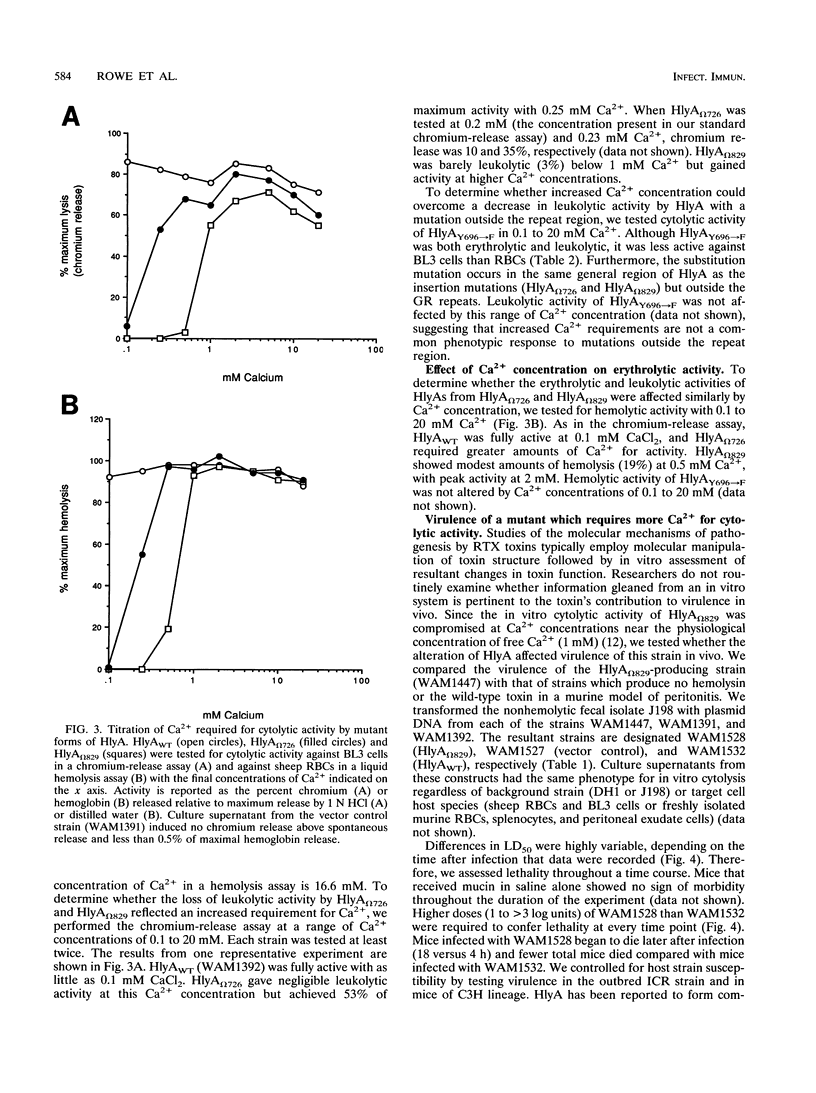
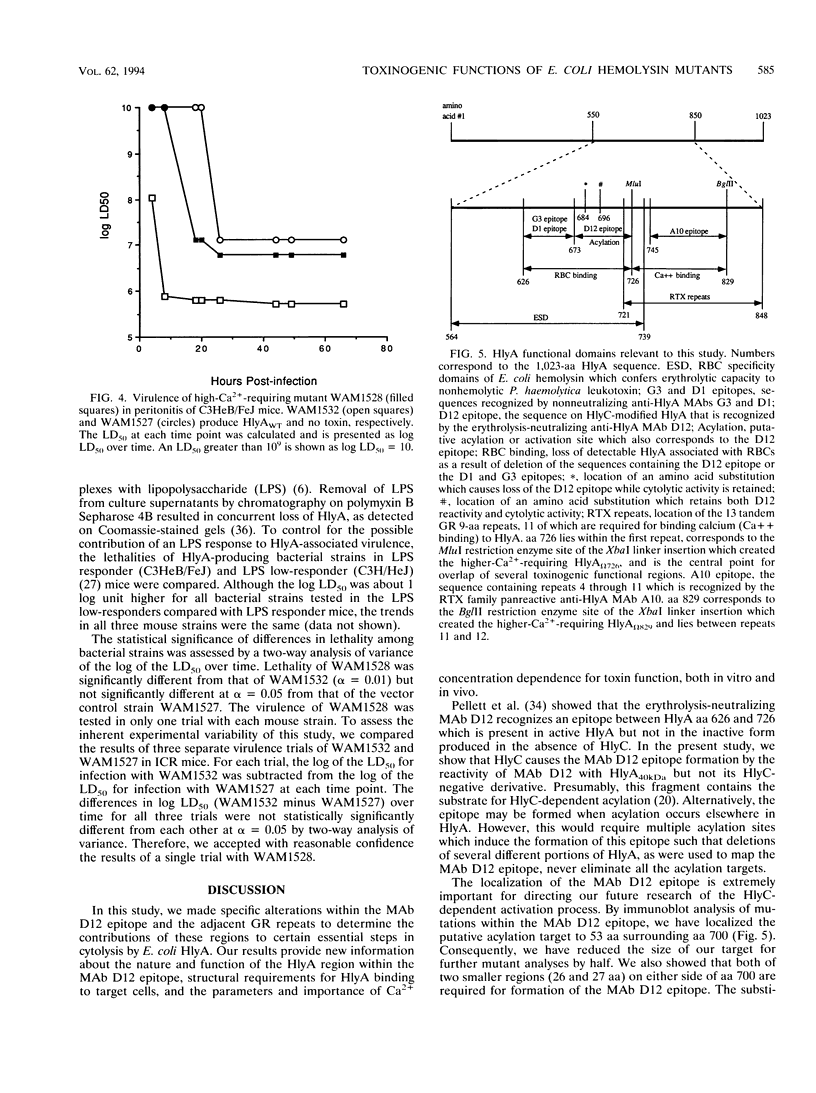
Amino acids (aa) 550 through 850 of the Escherichia coli hemolysin (HlyA) contain sequences important for several steps in cytolysis. These include the Ca(2+)-binding glycine-rich tandem repeats recognized by the monoclonal antibody A10, the putative HlyC-dependent acylation site that corresponds to the monoclonal antibody D12 epitope, and the erythrocyte specificity domain which confers erythrolytic activity to the Pasteurella haemolytica leukotoxin. To further investigate the toxinogenic functions associated with this region of HlyA, we constructed mutants in the hlyA sequences coding for the repeat region and the D12 epitope. Mutants were analyzed for anti-HlyA antibody reactivity, cytolytic activities, target cell binding, Ca2+ requirements, and virulence. The D12 epitope was mapped to aa 673 through 726, with portions of the epitope both amino terminal and carboxy terminal to aa 700. This region was necessary, but not sufficient, for toxin binding to erythrocytes. A substitution at aa 684 resulted in loss of the D12 epitope, while cytolytic activity was retained. The nature of the D12 epitope and its associated functions are discussed. The A10 epitope mapped to residues 745 through 829, corresponding to repeats 4 through 11. Insertions within the glycine-rich repeats resulted in mutant forms of HlyA which retained A10 reactivity but required increased Ca2+ for lytic activity. These in vitro effects on cytolysis corresponded to a significant decrease in HlyA-mediated virulence in mice. HlyA from one insertion mutant was able to associate with leukocyte membranes under conditions that were Ca2+ deficient for cytolysis. The role of the glycine-rich repeats and Ca2+ in HlyA activity are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluyut C. S., Simonson R. R., Bemrick W. J., Maheswaran S. K. Interaction of Pasteurella haemolytica with bovine neutrophils: identification and partial characterization of a cytotoxin. Am J Vet Res. 1981 Nov;42(11):1920–1926. [PubMed] [Google Scholar]
- Benz R., Schmid A., Wagner W., Goebel W. Pore formation by the Escherichia coli hemolysin: evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect Immun. 1989 Mar;57(3):887–895. doi: 10.1128/iai.57.3.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Snyder I. S. Chemical and immunological analysis of the complex structure of Escherichia coli alpha-hemolysin. J Bacteriol. 1985 Dec;164(3):1071–1080. doi: 10.1128/jb.164.3.1071-1080.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Snyder I. S. Composition of affinity-purified alpha-hemolysin of Escherichia coli. Infect Immun. 1986 Aug;53(2):435–437. doi: 10.1128/iai.53.2.435-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz W. T., Young R., Chang Y. F., Struck D. K. Deletion analysis resolves cell-binding and lytic domains of the Pasteurella leukotoxin. Mol Microbiol. 1990 Nov;4(11):1933–1939. doi: 10.1111/j.1365-2958.1990.tb02042.x. [DOI] [PubMed] [Google Scholar]
- Eberspächer B., Hugo F., Bhakdi S. Quantitative study of the binding and hemolytic efficiency of Escherichia coli hemolysin. Infect Immun. 1989 Mar;57(3):983–988. doi: 10.1128/iai.57.3.983-988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Welch R. A. Alterations of amino acid repeats in the Escherichia coli hemolysin affect cytolytic activity and secretion. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5269–5273. doi: 10.1073/pnas.85.14.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier C., Welch R. A. Identification of RTX toxin target cell specificity domains by use of hybrid genes. Infect Immun. 1991 Nov;59(11):4212–4220. doi: 10.1128/iai.59.11.4212-4220.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier C., Welch R. A. Nonreciprocal complementation of the hlyC and lktC genes of the Escherichia coli hemolysin and Pasteurella haemolytica leukotoxin determinants. Infect Immun. 1990 Mar;58(3):828–832. doi: 10.1128/iai.58.3.828-832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Gray L., Allietta M., Ehrmann I., Gordon V. M., Gray M. C. Adenylate cyclase toxin from Bordetella pertussis. Conformational change associated with toxin activity. J Biol Chem. 1991 Sep 15;266(26):17503–17508. [PubMed] [Google Scholar]
- Himmel M. E., Yates M. D., Lauerman L. H., Squire P. G. Purification and partial characterization of a macrophage cytotoxin from Pasteurella haemolytica. Am J Vet Res. 1982 May;43(5):764–767. [PubMed] [Google Scholar]
- Issartel J. P., Koronakis V., Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991 Jun 27;351(6329):759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Létoffé S., Wandersman C. Secretion of CyaA-PrtB and HlyA-PrtB fusion proteins in Escherichia coli: involvement of the glycine-rich repeat domain of Erwinia chrysanthemi protease B. J Bacteriol. 1992 Aug;174(15):4920–4927. doi: 10.1128/jb.174.15.4920-4927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N. E., Ziegler H. K. Role of lipopolysaccharide in induction of Ia expression during infection with gram-negative bacteria. Infect Immun. 1989 May;57(5):1556–1560. doi: 10.1128/iai.57.5.1556-1560.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney D. R., Chang Y. F., Young R., Struck D. K. Separable domains define target cell specificities of an RTX hemolysin from Actinobacillus pleuropneumoniae. J Bacteriol. 1992 Jan;174(1):291–297. doi: 10.1128/jb.174.1.291-297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menestrina G., Mackman N., Holland I. B., Bhakdi S. Escherichia coli haemolysin forms voltage-dependent ion channels in lipid membranes. Biochim Biophys Acta. 1987 Nov 27;905(1):109–117. doi: 10.1016/0005-2736(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pellett S., Boehm D. F., Snyder I. S., Rowe G., Welch R. A. Characterization of monoclonal antibodies against the Escherichia coli hemolysin. Infect Immun. 1990 Mar;58(3):822–827. doi: 10.1128/iai.58.3.822-827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982 Jan;35(1):91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., Simpson D. L., Sakurada S., Cranfield M., DiRienzo J., Slots J. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol Immunol. 1987 Sep;2(3):97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Wagner S. P., Welch R. A. A heterologous membrane protein domain fused to the C-terminal ATP-binding domain of HlyB can export Escherichia coli hemolysin. J Bacteriol. 1992 Nov;174(21):6771–6779. doi: 10.1128/jb.174.21.6771-6779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Shenker B. J., DiRienzo J. M., Malamud D., Taichman N. S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984 Feb;43(2):700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Hull R., Falkow S. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect Immun. 1983 Oct;42(1):178–186. doi: 10.1128/iai.42.1.178-186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect Immun. 1987 Sep;55(9):2183–2190. doi: 10.1128/iai.55.9.2183-2190.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]