Heart failure is a leading cause of mortality in the USA and Europe, and the ability to predict prognosis is essential for optimal allocation of treatments. Biomarkers offering prognostic information in patients with heart failure have recently entered practice. Although B‐type natriuretic peptide (BNP) is an established biomarker,1 uric acid may also have prognostic value.2,3
Increases in BNP results primarily from increasing cardiac filling pressures, whereas increases in uric acid are associated with increased vascular tone4 and depressed myocardial contractility via increased xanthine oxidase activity.5 Thus, uric acid, like BNP, could be associated with haemodynamic compromise in heart failure. We tested the hypothesis that increased uric acid levels are associated with worsening haemodynamic compromise in patients with heart failure independent of BNP.
Methods
Study patients were referred for right heart catheterisation between 1 January 2003 and 1 July 2004. Right atrial, pulmonary arterial and pulmonary capillary wedge (PCW) pressures were measured using a balloon‐tipped, flow‐directed catheter. Cardiac output was determined by thermodilution. Heart transplant recipients or patients currently receiving synthetic human BNP were excluded. Uric acid and N‐terminal proBNP (NT‐proBNP) levels were drawn during the right heart catheterisation. The study was approved by the Johns Hopkins Institutional Review Board.
Patients were divided into groups on the basis of the median serum uric acid and NT‐proBNP levels: low uric acid and low NT‐proBNP (n = 27), high uric acid and low NT‐proBNP (n = 24), low uric acid and high NT‐proBNP (n = 22), and high uric acid and high NT‐proBNP (n = 33). We compared haemodynamic parameters between groups using linear regression analysis (Stata V.8.0).
Results
The 106 patients had a median age of 53 years, were predominantly male (58%) and Caucasian (68%), with median ejection fraction of 20%. The median uric acid level was 481.7 μmol/l (8.1 mg/dl) and the NT‐proBNP level was 1583 ng/l. Most patients had ischaemic or idiopathic cardiomyopathy (63%) with New York Heart Association class III–IV symptoms (65%). Most patients were taking diuretics (85%), ACE inhibitors (71%) and β‐blockers (67%). A total of 10% patients were receiving intravenous inotropes, including dopamine, dobutamine and milrinone.
Serum uric acid did not correlate with NT‐proBNP (R = 0.03, p = 0.74), but did correlate with log NT‐proBNP (R = 0.24, p = 0.01). Both uric acid and NT‐proBNP positively correlated with right atrial, pulmonary arterial, and PCW pressures (data not shown). Uric acid, but not NT‐proBNP, also correlated positively with pulmonary vascular resistance index (PVRI; R = 0.24, p = 0.01) and inversely with indices of integrated cardiac performance, cardiac index (R = 0.18, p = 0.02) and left ventricular stroke work index (R = 0.27, p = 0.01), suggesting associations with vascular tone and myocardial function.
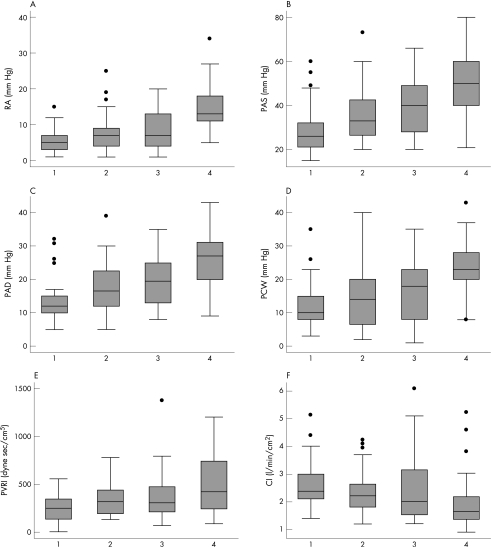
To determine whether uric acid levels offered additional clinical information independent of NT‐proBNP, patients were categorised as described above. Higher uric acid levels were associated with higher right atrial, pulmonary arterial and PCW pressures, lower PVRI and higher cardiac index regardless of NT‐proBNP levels (fig 1; p<0.001 for the trend in all comparisons). Higher levels of both uric acid and NT‐proBNP had a greater effect on right atrial, pulmonary arterial and PCW pressures, and PVRI than high levels of either biomarker alone. These trends persisted after adjusting for age, renal function, diuretic use and allopurinol use. Analyses performed excluding patients with gout, taking allopurinol or receiving inotropes yielded similar results (data not shown).
Figure 1 Boxplots of haemodynamic parameters in groups defined by uric acid (UA) and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels. Group 1 is low UA and low NT‐proBNP, group 2 is high UA and low NT‐proBNP, group 3 is low UA and high NT‐proBNP, and group 4 is high UA and high NT‐proBNP. The median value is indicated by the central horizontal line; the lower and upper quartiles by the corresponding ends of the box; the 10th and 90th centiles by the bars; and circles show individual outliers. With higher levels of uric acid and NT‐proBNP, there is higher right atrial (RA) pressure (panel A), pulmonary arterial systolic (PAS) pressure (panel B), pulmonary arterial diastolic (PAD) pressure (panel C), pulmonary capillary wedge (PCW) pressure (panel D), higher pulmonary vascular resistance index (PVRI; panel E) and lower cardiac index (CI; panel F). In all cases, the p value for trend is <0.001 except for CI where p = 0.03. High uric acid is ⩾476 μmol/l. High NT‐proBNP is ⩾1500 ng/l.
Discussion
This study shows that high levels of uric acid are associated with worse haemodynamic profiles regardless of NT‐proBNP and after adjusting for age, renal function, diuretic use and allopurinol use. Furthermore, increases in both uric acid and NT‐proBNP are associated with worse haemodynamic profiles than increases in either alone. The previously unanticipated additive association of increases in uric acid and NT‐proBNP on right atrial, pulmonary arterial and PCW pressures suggests that the two may have independent and complementary roles in heart failure pathogenesis. In addition, uric acid correlated with PVRI and negatively correlated with cardiac index suggesting associations with vascular tone and cardiac performance, providing a potential basis for independent predictive capacity.
Although their exact roles in heart failure pathogenesis can only be elucidated from controlled animal studies, and although this association does not imply causation, it is still reasonable to explore potential mechanisms underlying these findings. BNP is increased in an adaptive response to increased ventricular filling pressures.1 Uric acid, on the other hand, is increased in what is probably a maladaptive response as a marker of increased xanthine oxidase activity.5 An understanding of the adaptive response of BNP in heart failure has led to the use of synthetic human BNP and nesiritide in the treatment of decompensated heart failure, and the use of BNP or pro‐BNP as a biomarker of the presence and severity of disease. Accordingly, uric acid has the potential to serve as a novel biomarker,2,3 and contributes to the hypothesis that xanthine oxidase inhibition may be a therapeutic principal for heart failure.
This study is limited by small sample size, which limits the ability to detect a true association if one exists. However, even in this small cohort, independent effects of uric acid and NT‐proBNP on haemodynamic parameters are detected. Although small samples may also prevent generalisability, we studied diverse patients, suggesting applicability of our results to a wide spectrum of heart failure.
We also examined haemodynamic rather than clinical end points. However, clinical end points have already been studied for both uric acid and BNP; our goals were to describe the relationship between uric acid, BNP and haemodynamic compromise, and to further elucidate potential mechanisms by which uric acid contributes to increased mortality in heart failure. Nevertheless, we emphasise that our results are observational and that association does not imply causation; there may be unmeasured confounders that affected our results.
In summary, both uric acid and NT‐proBNP are independently associated with increased filling and pulmonary arterial pressures and pulmonary vascular resistance such that an increase in both results in worse haemodynamic profiles than an increase in either one alone. This study may explain the previously observed association between uric acid and increased mortality in patients with heart failure, offers therapeutic insight into the potential role of xanthine oxidase in heart failure and illustrates the value of uric acid, in addition to NT‐proBNP, as a biomarker to assess haemodynamic decompensation in patients with heart failure.
Acknowledgements
This research was supported by NIH Grant 5RO1‐HL‐065455 (JMH). JMH is a recipient of the Paul Beeson Physician Faculty Scholars in Aging Research Award.
Abbreviations
BNP - B‐type natriuretic peptide
NT‐proBNP - N‐terminal proBNP
PCW - pulmonary capillary wedge
PVRI - pulmonary vascular resistance index
Footnotes
Competing interests: JMH is a paid consultant at Cardiome Pharma Corporation, a manufacturer of oxypurinol. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. Cardiome Pharma Corporation had no involvement in the study design, data collection or analysis, writing of the report, or in the decision to submit the paper for publication.
References
- 1.McDonagh T A, Robb S D, Murdoch D R.et al Biochemical detection of left‐ventricular systolic dysfunction. Lancet 19983519–13. [DOI] [PubMed] [Google Scholar]
- 2.Levy W C, Mozaffarian D, Linker D T.et al The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 20061131424–1433. [DOI] [PubMed] [Google Scholar]
- 3.Anker S D, Doehner W, Rauchhaus M.et al Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 20031071991–1997. [DOI] [PubMed] [Google Scholar]
- 4.Doehner W, Rauchhaus M, Florea V G.et al Uric acid in cachectic and noncachectic patients with chronic heart failure: relationship to leg vascular resistance. Am Heart J 2001141792–799. [DOI] [PubMed] [Google Scholar]
- 5.Cappola T P, Kass D A, Nelson G S.et al Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 20011042407–2411. [DOI] [PubMed] [Google Scholar]