Abstract
Objective
To determine the effect of plasma glucose lowering on coronary circulatory function in type 2 diabetes mellitus.
Methods
Twenty patients with type 2 diabetes and 18 weight‐matched controls were studied. At baseline, myocardial blood flow (MBF) was measured with [13N]ammonia and positron emission tomography at rest, during cold pressor testing (CPT), and during adenosine hyperaemia. In diabetic patients, MBF and blood chemistry were analysed again after 3 months of glucose‐lowering treatment with glyburide and metformin.
Results
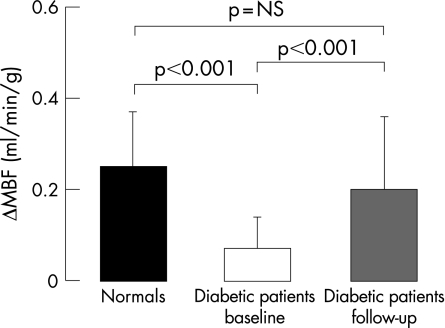
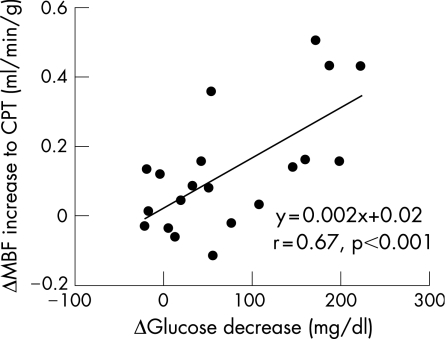
Although hyperaemic MBF did not differ significantly between the patients and controls (1.81 (0.38) v 1.97 (0.43) ml/min/g; mean (SD)), the CPT‐induced MBF increase (ΔMBF) was significantly less in diabetic patients than in controls (0.07 (0.07) v 0.25 (0.12) ml/min/g; p<0.001). Treatment with glyburide and metformin significantly decreased plasma glucose concentrations from 207 (76) to 134 (52) mg/dl (p<0.001). This decrease in plasma glucose was paralleled by a significant increase in ΔMBF in response to CPT (0.20 (0.16) from 0.07 (0.07) ml/min/g; p<0.001), which tended to be lower than in controls at baseline (0.20 (0.16) v 0.25 (0.12) ml/min/g; p = NS). The decrease in plasma glucose concentrations correlated significantly with the improvement in ΔMBF in response to CPT (r = 0.67, p<0.01).
Conclusions
Type 2 diabetes mellitus is associated with abnormal MBF response to CPT, which can be significantly improved by euglycaemic control with glyburide and metformin. The close association between the decrease in plasma glucose concentration and the improvement in coronary vasomotor function in response to CPT suggests a direct adverse effect of raised plasma glucose concentration on diabetes‐related coronary vascular disease.
Keywords: coronary disease, cold pressor testing, type 2 diabetes, endothelium, myocardial blood flow, positron emission tomography
The prevalence of diabetes in the US according to the American Diabetes Association rose from 1–2% at ages 29–39 to 18–20% at ages 60–75.1 As diabetes is recognised as a major risk factor for cardiovascular morbidity and mortality,2 its increasing incidence is of considerable public health concern. The exact mechanisms underlying diabetes‐related coronary vascular disease are still incompletely understood. An impairment of coronary vasomotor function has been shown to precede and accompany the development and progression of atherosclerosis.3,4 Functional abnormalities related to insulin resistance and hyperglycaemia,5 associated with pro‐atherosclerotic and pro‐thrombotic effects, may therefore reflect an important mechanistic link between coronary vasomotor dysfunction and the excess cardiac morbidity and mortality in type 2 diabetes mellitus.1,3 Several mechanisms are involved in the diabetes‐related endothelial dysfunction.6 Besides insulin resistance itself, raised plasma free fatty acid concentration together with increased plasma triglycerides and small, dense low‐density lipoprotein (LDL) bodies may adversely affect intracellular signalling of nitric oxide synthase activity, thereby reducing endothelial production and release of nitric oxide. Hyperglycaemia is associated with increased production of reactive oxygen species which interact with nitric oxide to form peroxynitrite and, thereby, diminish the bioavailability of endothelium‐derived nitric oxide, which is assumed to be in part responsible for the observed abnormal vasomotor function.6 On the other hand, raised plasma glucose concentrations in patients with diabetes may also cause endothelial dysfunction by altering endothelium‐derived hyperpolarising factor activity, particularly in smaller arteries and arterioles.7,8
Increases in glucose concentration in healthy subjects induced by glucose infusion have been shown to produce alterations in endothelium‐related forearm blood flow.9 Furthermore, glucose lowering in patients with diabetes through acute and long‐term insulin administration or dietary control and anti‐diabetic agents may lead to an improvement in endothelium‐mediated forearm blood flow.10,11
The aim of this study was therefore to determine whether glucose lowering with anti‐diabetic agents beneficially influences diabetes‐related abnormalities of the coronary circulatory function.
Methods
Study design
In 20 patients with type 2 diabetes, but without traditional coronary risk factors such as smoking, hypercholesterolaemia and arterial hypertension, coronary vasoreactivity was assessed by measuring myocardial blood flow (MBF) and its responses to sympathetic stimulation with cold pressor testing (CPT) and pharmacologic vasodilatation. MBF values were compared with those in 18 healthy subjects matched for age and weight. After the baseline measurements, the diabetic patients were placed on glucose‐lowering drugs for 3 months, after which blood chemistry analysis and MBF measurements were repeated. The study was approved by the UCLA Institutional Review Board, and each participant signed the approved informed consent form.
Study population
Twenty patients (nine male, 11 female; mean (SD) age 53 (7) years) with type 2 diabetes were enrolled (table 1). At initial screening visits before the baseline positron emission tomography studies, type 2 diabetes mellitus was diagnosed by standard criteria including raised fasting plasma glucose concentrations (>126 mg/dl) on at least two occasions and raised HbA1c concentrations.12 Because of limited access to healthcare services and drugs, the patients enrolled in this study had not been receiving regular anti‐diabetic drugs, but had attempted to control diabetes by diet, weight loss, and physical activity. None of the participants was receiving glucose‐lowering drugs at the time of inclusion in the study protocol. The patients reported a mean (SD) duration of their diabetes of 26 (16) months (range 6–48). None had a family history of premature coronary artery disease, and none had a history of coronary or peripheral vascular or other diseases. All the women were postmenopausal (cessation of menses ⩾1 year), and none was on hormone replacement therapy. Physical examination showed no abnormalities in any of the patients, and all had a normal resting electrocardiogram and normal heart rate and blood pressure (<135/80 mm Hg), on at least two occasions. Total and LDL plasma cholesterol concentrations were less than 240 mg/dl and 155 mg/dl, respectively. None of the patients currently smoked or had in the past, and none was taking any drugs.
Table 1 Characteristics of and laboratory findings for the controls and patients with type 2 diabetes at baseline and 3‐month follow‐up.
| Controls | Patients | ||
|---|---|---|---|
| Baseline | Follow‐up | ||
| Number | 18 | 20 | 20 |
| Age (years) | 49 (7) | 53 (7) | – |
| Body mass index (kg/m2) | 27 (4) | 30 (5) | 29 (4) |
| HbA1c (%) | 5.3 (0.3) | 10.0 (2.7)* | 8.5 (1.4)† |
| Fasting plasma concentrations | |||
| Glucose (mg/dl) | 84 (13) | 207 (76)* | 134 (52)† |
| Lactic acid (mg/dl) | 13.1 (3.1) | 16.0 (3.8) | 17.1 (4.5) |
| Free fatty acids (mmol/l) | 0.50 (0.28) | 0.78 (0.36)* | 0.61 (0.47) |
| Insulin (μU/ml) | 11 (10) | 8.4 (7) | 13 (12) |
| Total cholesterol (mg/dl) | 178 (36) | 214 (46)* | 214 (42) |
| LDL cholesterol (mg/dl) | 107 (24) | 124 (35) | 128 (35) |
| HDL cholesterol (mg/dl) | 43 (12) | 44 (10) | 48 (9) |
| Triglycerides (mg/dl) | 170 (129) | 244 (205) | 212 (141) |
HbA1c, haemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Values are mean (SD).
*p<0.04 v controls, †p<0.05 v baseline in diabetes (Wilcoxon rank test).
Measurement of MBF and assessment of coronary vasoreactivity
MBF was measured with [13N]ammonia, serial image acquisition by positron emission tomography (ECAT EXACT HR+; CTI/Siemens, Knoxville, Tennessee, USA) and a two‐compartment tracer kinetic model as described previously.5,13 From the last 15‐min transaxial image, reoriented short‐axis and long‐axis myocardial slices and the corresponding polar map were submitted to visual and semiquantitative analysis. All study participants had normal homogeneous [13N]ammonia tracer uptake. MBF (ml/g/min) was measured at rest, during CPT (reflecting predominantly endothelium‐dependent vasomotion), and during adenosine‐stimulated hyperaemia (140 μg/kg/min; reflecting predominantly endothelium‐independent vasomotion).5,13 Regional MBF values from the three major coronary artery territories on the polar map were averaged to yield mean MBF.
Heart rate, blood pressure, and a 12‐lead electrocardiogram were recorded continuously during each MBF measurement. From the mean heart rate and systolic blood pressure recorded during the first 2 min of image acquisition, the rate–pressure product (RPP) was determined as an index of cardiac work. Changes in MBF from rest to CPT (ΔMBF) were expressed in ml/min/g. Further, to account for intraindividual and interindividual differences in MBF and its response to cold, MBF was normalised to the RPP, and thus myocardial work (averaged during the first 2 min of image acquisition; MBF divided by RPP multiplied by 10 000).
Study protocol
After the baseline measurements of MBF and blood chemistry, the 20 patients with type 2 diabetes were started on glucose‐lowering treatment. Treatment was begun with oral glyburide at a dose of 10 mg/day. If, after 10 days, fasting glucose concentrations remained above 126 mg/dl, the dose was increased to 10 mg twice a day (total of 20 mg/day). If, after an additional 20 days, plasma glucose concentrations remained raised (>126 mg/dl), metformin, at a dose of 500 mg twice a day (total of 1000 mg/day), was added to the glyburide. Patients and immediate family members were instructed to contact one of the investigators in instances of hypoglycaemia or other adverse reactions.
Statistical analysis
Data are presented as mean (SD) for quantitative and absolute frequencies for qualitative variables. The appropriate Wilcoxon rank test for independent or paired samples was used (SAS Institute). A comparison of CPT‐induced ΔMBF and adenosine‐induced MBF between the different groups was performed by one‐way analysis of variance, followed by Scheffe's multiple comparison test. Correlations between selected variables were estimated by Spearman correlation coefficients (r). All test procedures were two‐sided, and p<0.05 was considered to indicate significance.
Results
Clinical characteristics at baseline
Total plasma cholesterol and free fatty acid concentrations were significantly higher in the patients with diabetes than in the controls, and LDL cholesterol, triglyceride and lactic acid concentrations tended to be higher (table 1). Insulin concentrations tended to be lower in the diabetic patients than in the controls.
MBF measurements at baseline
At rest, heart rate, systolic and diastolic blood pressures and RPPs were nearly identical in the two study groups (table 2). Furthermore, MBF at rest was similar in the controls and diabetic patients.
Table 2 Haemodynamic data and myocardial blood flow in the controls and patients with type 2 diabetes at baseline and 3‐month follow‐up.
| Controls | Patients | Patients | |
|---|---|---|---|
| Baseline | Follow‐up | ||
| Rest | |||
| Heart rate (beats/min) | 66 (8) | 69 (11) | 69 (9) |
| SBP (mm Hg) | 128 (17) | 137 (23) | 131 (22) |
| DBP (mm Hg) | 78 (10) | 76 (9) | 76 (9) |
| MAP (mm Hg) | 94.7 (12.1) | 96.9 (10.4) | 94.6 (11.3) |
| RPP (mm Hg/min) | 8489 (1613) | 9490 (2404) | 9151 (2287) |
| MBF (ml/min/g) | 0.72 (0.16) | 0.75 (0.24) | 0.67 (0.17) |
| Adenosine‐induced hyperaemia | |||
| Heart rate (beats/min) | 88 (13) | 92 (14) | 96 (15) |
| SBP (mm Hg) | 126 (13) | 131 (23) | 132 (21) |
| DBP (mm Hg) | 77 (7) | 76 (9) | 76 (9) |
| MAP (mm Hg) | 93.6 (7.8) | 91.2 (11.3) | 94.5 (12.2) |
| RPP (mm Hg/min) | 11175 (2030) | 12130 (3303) | 12717 (3336) |
| MBF (ml/min/g) | 1.97 (0.43) | 1.81 (0.38) | 1.87 (0.46) |
| Cold pressor testing | |||
| Heart rate (beats/min) | 75 (11) | 74 (10) | 74 (10) |
| SBP (mm Hg) | 154 (19) | 164 (32) | 159 (30) |
| DBP (mm Hg) | 90 (11) | 89 (11) | 89 (11) |
| MAP (mm Hg) | 111.3 (13.5) | 114.5 (16.4) | 111.9 (15.0) |
| RPP (mm Hg/min) | 11546 (2442) | 12198 (3197) | 11860 (3063) |
| MBF (ml/min/g) | 0.96 (0.25) | 0.81 (0.24) | 0.87 (0.28) |
| ΔRPP (mm Hg/min) | 3058 (1832) | 2709 (1727) | 2710 (1444) |
| ΔMBF (ml/min/g) | 0.25 (0.12) | 0.07 (0.07)* | 0.20 (0.16)† |
SBP, Systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; RPP, rate–pressure product (heart rate × systolic blood pressure); MBF, myocardial blood flow.
Values are mean (SD).
*p<0.001 v controls, †p<0.001 v baseline in diabetes (Wilcoxon rank test).
During adenosine‐induced hyperaemia, heart rates had increased significantly from rest but were similar in the two groups (table 2), whereas systolic and diastolic blood pressures had remained unchanged from rest. As given in table 2, MBF during hyperaemia in patients with type 2 diabetes tended to be non‐significantly lower than in the controls. When the hyperaemic MBFs were related to the mean arterial blood pressure in order to account for interindividual variations in coronary driving pressure, the resulting estimates of coronary vascular resistance (mean arterial blood pressure/MBF) were similar for the two study groups (50.1 (12.8) and 51.9 (9.8) mm Hg/ml/min/g).
During CPT, heart rate and systolic and diastolic blood pressure in both study groups were significantly higher than at rest (table 2). Although the corresponding RPPs were comparable in the two groups, absolute MBFs during CPT tended to be lower in the diabetic patients than in the controls (p = 0.06) (table 2). When adjusted for RPP, the normalised MBF during CPT was significantly lower in the diabetic than in the control group (0.67 (0.15) v 0.82 (0.20) ml/min/g; p<0.01). Moreover, mean increases in RPP from rest to CPT (defined as ΔRPP, the difference between CPT and rest) were similar in the two study groups (table 2), whereas the corresponding change in MBF from rest to CPT (ΔMBF) was significantly less in the diabetic patients than in the controls (p<0.001). Thus, despite comparable increases in cardiac work with CPT, the increase in blood flow was significantly attenuated in the patients with diabetes (table 2 and fig 1).
Figure 1 Change in myocardial blood flow (ΔMBF) in response to cold pressor testing in controls and patients with type 2 diabetes at baseline and follow‐up.
Follow‐up studies after glucose lowering in patients with diabetes
After treatment with glyburide or glyburide/metformin for 3 months, all patients returned for follow‐up measurements of MBF and blood chemistry (tables 1 and 2). Plasma glucose and HbA1c concentrations had significantly declined from baseline. At follow‐up, insulin concentrations tended to be higher than at baseline, although this was not statistically significant. Free fatty acid and triglyceride concentrations tended to be lower at follow‐up than at baseline, and high‐density lipoprotein cholesterol had increased.
At rest, heart rate, systolic and diastolic blood pressures and RPPs were similar at follow‐up and baseline (table 2). Further, resting MBF on repeat assessment did not differ significantly. Adenosine‐stimulated MBF at follow‐up tended to be higher than at baseline but not significantly. Further, the minimal coronary vascular resistance during adenosine stimulation was unchanged (51.9 (9.8) and 52.3 (9.6) mm Hg/ml/min/g).
During CPT, heart rate, systolic and diastolic blood pressure significantly increased at baseline and follow‐up so that the RPP, and thus cardiac work, was similar at baseline and follow‐up (table 2). The ΔMBF in response to CPT was significantly greater than that at baseline, but still tended to be less than that in the control group (table 2, fig 1). In addition, normalised MBF during CPT was significantly higher at follow‐up than at baseline (0.75 (0.15) v 0.67 (0.15) ml/min/g; p<0.001 ) but again tended to be lower than in the control group (0.75 (0.15) v 0.82 (0.20) ml/min/g; p = NS).
Comparison of MBF responses and changes in plasma glucose concentrations
Decreases in fasting plasma glucose concentrations from baseline to follow‐up varied considerably. Fourteen diabetic patients had achieved euglycaemic values of <126 mg/dl, whereas glucose concentrations in the remaining six patients ranged from 150 to 234 mg/dl. The change in fasting plasma glucose concentrations from baseline to follow‐up was expressed as the difference in glucose concentrations at baseline and follow‐up, and was compared with the improvement in the flow response to CPT (again defined as the difference in ΔMBFs between follow‐up and baseline). This decrease in plasma glucose concentration correlated significantly with an improvement in the flow response to cold (r = 0.67, p<0.01; fig 2). In addition, after the exclusion of the six patients in whom post‐treatment glucose concentrations remained >126 mg/dl, the correlation remained significant (r = 0.59, p<0.02).
Figure 2 Correlation of the change in myocardial blood flow (ΔMBF) induced by cold pressor testing (CPT) and change in fasting plasma glucose concentrations as defined as difference in ΔMBF and Δglucose decrease between follow‐up and baseline.
Discussion
The novel finding of the current study is that the abnormal endothelium‐related coronary flow responses to CPT in patients with type 2 diabetes can be significantly improved by euglycaemic control with glyburide and metformin. Moreover, the close association between the decrease in plasma glucose concentration and the improvement in endothelium‐related coronary vasomotor function suggests a direct adverse effect of raised plasma glucose on diabetes‐related coronary vascular disease.
Metabolic profiles and alterations with glucose‐lowering treatment
Patients with type 2 diabetes mellitus but without traditional coronary risk factors such as smoking, hypercholesterolaemia or arterial hypertension were studied in order to separate possibly confounding effects of these risk factors on coronary circulatory function.14 Surprisingly, plasma insulin concentrations tended to be lower in the diabetic patients than in controls matched for age and weight. It is conceivable that overweight controls had a pre‐diabetic state of impaired glucose tolerance associated with an increase in plasma insulin concentrations.5 Furthermore, the lower plasma insulin concentrations in the patients may also reflect a later stage of diabetic disease at which insulin secretion had already sufficiently declined.15 However, of the 20 diabetic patients with very high glucose concentrations, the 3‐month course of glyburide or glyburide/metformin achieved euglycaemic control in 14 but not in six. This may explain in part why the decrease in plasma HbA1c concentration was not as pronounced as might have been expected. The reason for the inadequate and/or delayed glucose lowering in the diabetic patients remains unknown, but it could be related to as yet unknown metabolic, genetic or vascular factors6 or to limited patient compliance.16 In contrast with the significant decrease in plasma glucose concentrations, total and LDL cholesterol concentrations at follow‐up had not changed significantly from baseline. On the other hand, free fatty acid and triglyceride concentrations tended to be lower at follow‐up than at baseline, and high‐density lipoprotein cholesterol and insulin concentrations tended to be higher. The latter changes in the metabolic profile may have contributed to the observed improvement in coronary vasomotor function in the diabetic patients.6
MBF responses to CPT and adenosine
Exposure to cold prompts an α‐adrenergically mediated vasoconstriction of the coronary vascular smooth muscle cells, which, under normal conditions, is offset by a flow‐mediated and, in part, direct adrenergically induced endothelium‐dependent vasodilation.17 In the presence of endothelial dysfunction, however, the vasoconstrictor effect may remain largely unopposed.17 Thus flow responses to CPT represent the net effect of endothelium‐related vasodilator effects and vasoconstrictor responses of the coronary vascular smooth muscle cells. Flow increases in response to sympathetic stimulation with CPT have been reported to be severely diminished or even absent in the presence of mild coronary artery disease or risk factors for coronary artery disease, as well as in diabetes mellitus as a cardiovascular disease equivalent.13,17,18
The total vasodilator capacity, as reflected by the magnitude of the adenosine‐stimulated and predominantly endothelium‐independent hyperaemic flow increase, in the diabetic patients in this study tended to be somewhat lower, although not significantly so, than in the control group. This is at variance with previous findings for type 1 and type 2 diabetes mellitus with significantly reduced hyperaemic blood flow.19,20 The reason for this discordant observation is not known, but may be related to differences in patient characteristics, differences in the duration of diabetes, and/or differences in the state of diabetes, including diabetic microangiopathy and vascular smooth muscle involvement.21 It is possible that, in the diabetic patients in the present study, vascular alterations were largely confined to the endothelium and had not yet affected vascular smooth muscle function, as observed also with forearm blood flow measurements in type 2 diabetes and for the coronary circulation in people with increasing body weight.13,22 In more advanced stages of diabetes, higher oxidative stress burden and/or greater abnormalities in LDL subfractions and LDL oxidation imposed on the vascular wall may also directly affect smooth muscle cell function and thus lead to impairment of endothelium‐independent vasodilation.14 The recently reported relationship between reductions in total vasodilator capacity and structural alterations of the arterial wall in more advanced states of diabetes mellitus supports this possibility.23
Effects of glucose‐lowering treatment
An important finding of this study is the beneficial effect of a 3‐month glucose‐lowering treatment with glyburide alone or in combination with metformin on coronary vasomotion in type 2 diabetes. Whereas, at baseline, flow responses to cold were severely diminished in patients with type 2 diabetes compared with those in non‐diabetic and weight‐matched controls, the flow response after treatment had significantly improved in the patients. The important role of glucose lowering is supported further by the observed inverse and statistically significant correlation between reductions in plasma glucose concentration and increases in the flow response to cold from baseline to follow‐up. This correlation implicates glucose lowering as an important determinant of this improvement. Possible mechanisms may involve a reduction in hyperglycaemia‐mediated formation of reactive oxygen species and inactivation of protein kinase C associated with an increase in the bioavailability of endothelium‐derived nitric oxide6 and an increase in endothelium‐derived hyperpolarising factor activity.7,24
Although not assessed in this study, diabetic autonomic neuropathy was unlikely to have accounted for the diminished flow response for several reasons. Basal heart rate, blood pressure and coronary flow in the patients were normal and not elevated as observed in some but not all previous investigations in patients with diabetic neuropathy and abnormalities in cardiac sympathetic innervation.19,25 Secondly, had the abnormal flow response to cold resulted from a defective efferent cardiac sympathetic innervation, it is unlikely that it would have normalised over the relatively short time period of only 3 months of glucose lowering when compared with the modest improvement in regional cardiac innervation observed previously over a much longer time period.26
The observed improvement in the flow response to cold in the patients in this study is certainly unlikely to be explained by the glucose‐lowering effects only of glyburide and metformin. Insulin secretagogues such as sulphonylureas raise basal and post‐prandial insulin secretion.27 Although sulphonylureas have little, if any, effect on the tissue sensitivity to insulin, they may have contributed to the improvement in the flow response to CPT through insulin‐induced and endothelium‐dependent vasodilation mediated by nitric oxide.28 Furthermore, metformin lowers plasma glucose concentrations predominantly through its insulin‐sensitising properties. The effects of both stimulation of insulin secretion by glyburide and greater vascular insulin sensitivity produced by metformin may therefore also have accounted in part for the endothelium‐related flow increase in response to CPT.10 In addition, this improvement also probably resulted from direct stimulatory effects of glyburide and metformin on endothelium‐derived hyperpolarising factor activity.29,30
Conclusions
In this study, we have shown an improvement in abnormal coronary vasomotor function related to glucose‐lowering treatment in type 2 diabetes mellitus. The close association between the decrease in plasma glucose concentration and the improvement in coronary vasomotor function provides first in vivo evidence of a direct adverse effect of raised plasma glucose concentration on diabetes‐related coronary vascular disease. As abnormalities in vasomotor function contain predictive information for future cardiovascular events,3 it remains to be established whether anti‐diabetic medical and/or behavioural interventions related to weight, diet and physical activity2,16 aiming to restore coronary vasomotor function in patients with type 2 diabetes will indeed improve the clinical outcome.
Abbreviations
CPT - cold pressor testing
LDL - low‐density lipoprotein
MBF - myocardial blood flow
RPP - rate–pressure product
Footnotes
Financial support: Supported in part by Research Grant HL 33177, National Heart, Lung and Blood Institute, Bethesda, MD, USA.
Competing interests: None.
The study was approved by the UCLA Institutional Review Board.
References
- 1.Harris M I, Flegal K M, Cowie C C.et al Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 199821518–524. [DOI] [PubMed] [Google Scholar]
- 2.Fisher M. Diabetes and atherogenesis. Heart 200490336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerman A, Zeiher A M. Endothelial function: cardiac events. Circulation 2005111363–368. [DOI] [PubMed] [Google Scholar]
- 4.Schindler T H, Nitzsche E U, Schelbert H R.et al Positron emission tomography‐measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol 2005451505–1512. [DOI] [PubMed] [Google Scholar]
- 5.Prior J O, Quinones M J, Hernandez‐Pampaloni M.et al Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation 20051112291–2298. [DOI] [PubMed] [Google Scholar]
- 6.Hink U, Li H, Mollnau H.et al Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 200188E14–E22. [DOI] [PubMed] [Google Scholar]
- 7.Vanhoutte P M, Miller V M. Alpha 2‐adrenoceptors and endothelium‐derived relaxing factor. Am J Med 1989871S–5S. [DOI] [PubMed] [Google Scholar]
- 8.Nelson M T, Conway M A, Knot H J.et al Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. J Physiol 1997502(Pt 2)259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams S B, Goldfine A B, Timimi F K.et al Acute hyperglycemia attenuates endothelium‐dependent vasodilation in humans in vivo. Circulation 1998971695–1701. [DOI] [PubMed] [Google Scholar]
- 10.Mather K J, Verma S, Anderson T J. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol 2001371344–1350. [DOI] [PubMed] [Google Scholar]
- 11.Vehkavaara S, Yki‐Jarvinen H. 3.5 years of insulin therapy with insulin glargine improves in vivo endothelial function in type 2 diabetes. Arterioscler Thromb Vasc Biol 200424325–330. [DOI] [PubMed] [Google Scholar]
- 12.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 13.Schindler T H, Cardenas J, Prior J O.et al Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol 2006471188–1195. [DOI] [PubMed] [Google Scholar]
- 14.Tan K C, Ai V H, Chow W S.et al Influence of low density lipoprotein (LDL) subfraction profile and LDL oxidation on endothelium‐dependent and independent vasodilation in patients with type 2 diabetes. J Clin Endocrinol Metab 1999843212–3216. [DOI] [PubMed] [Google Scholar]
- 15.Ferrannini E, Gastaldelli A, Miyazaki Y.et al beta‐Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 200590493–500. [DOI] [PubMed] [Google Scholar]
- 16.Cradock S. Helping patients to improve self management of diabetes. Heart. 2004;90: iv36–8; discussion iv39–40, (Suppl 4) [DOI] [PMC free article] [PubMed]
- 17.Schindler T H, Nitzsche E U, Olschewski M.et al HR. PET‐measured responses of MBF to cold pressor testing correlate with indices of coronary vasomotion on quantitative coronary angiography. J Nucl Med 200445419–428. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann P A, Camici P G. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med 20054675–88. [PubMed] [Google Scholar]
- 19.Pop‐Busui R, Kirkwood I, Schmid H.et al Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol 2004442368–2374. [DOI] [PubMed] [Google Scholar]
- 20.Nitenberg A, Valensi P, Sachs R.et al Impairment of coronary vascular reserve and ACh‐induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes 1993421017–1025. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson P, Celermajer D S, Donald A E.et al Impaired vascular reactivity in insulin‐dependent diabetes mellitus is related to disease duration and low density lipoprotein cholesterol levels. J Am Coll Cardiol 199628573–579. [DOI] [PubMed] [Google Scholar]
- 22.Economides P A, Caselli A, Tiani E.et al The effects of atorvastatin on endothelial function in diabetic patients and subjects at risk for type 2 diabetes. J Clin Endocrinol Metab 200489740–747. [DOI] [PubMed] [Google Scholar]
- 23.Sundell J, Janatuinen T, Ronnemaa T.et al Diabetic background retinopathy is associated with impaired coronary vasoreactivity in people with Type 1 diabetes. Diabetologia 200447725–731. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald S M, Kemp‐Harper B K, Tare M, Parkington H C. Role of endothelium‐derived hyperpolarizing factor in endothelial dysfunction during diabetes. Clin Exp Pharmacol Physiol 200532482–487. [DOI] [PubMed] [Google Scholar]
- 25.Di Carli M F, Bianco‐Batlles D, Landa M E.et al Effects of autonomic neuropathy on coronary blood flow in patients with diabetes mellitus. Circulation 1999100813–819. [DOI] [PubMed] [Google Scholar]
- 26.Stevens M J, Raffel D M, Allman K C.et al Regression and progression of cardiac sympathetic dysinnervation complicating diabetes: an assessment by C‐11 hydroxyephedrine and positron emission tomography. Metabolism 19994892–101. [DOI] [PubMed] [Google Scholar]
- 27.Feher M D. Diabetes: preventing coronary heart disease in a high risk group. Heart 200490(Suppl 4)iv18–iv21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundell J, Knuuti J. Insulin and myocardial blood flow. Cardiovasc Res 200357312–319. [DOI] [PubMed] [Google Scholar]
- 29.Gomes M B, Cailleaux S, Tibirica E. Metformin prevents the impairment of endothelium‐dependent vascular relaxation induced by high glucose challenge in rabbit isolated perfused kidneys. Naunyn Schmiedebergs Arch Pharmacol 200537224–30. [DOI] [PubMed] [Google Scholar]
- 30.Feletou M, Vanhoutte P M. The alternative: EDHF. J Mol Cell Cardiol 19993115–22. [DOI] [PubMed] [Google Scholar]