Abstract
Background
Given the high cardiac mortality of renal transplant recipients, identification of high‐risk patients is important to offer appropriate treatment before transplantation.
Aim
To determine patients with high mortality after renal transplantation despite selection according to current criteria.
Methods
Preoperative parameters were collected from 203 renal transplant recipients over a follow‐up time of 3.6 (1.9) years. The primary end point was all‐cause mortality.
Results
22 deaths (11%) and 12 cardiac failures (6%) were observed. Non‐survivors were older (p⩽0.001), had larger left ventricular end‐systolic diameter (LVSD) (p⩽0.001) and end‐diastolic diameter (p = 0.002), and lower ejection fraction (p⩽0.001). Left ventricular mass index (p = 0.001), maximal wall thickness (p = 0.006) and the proportion with mitral annular calcification (p = 0.001) were significantly higher in the non‐survivors. The risk factors for ischaemic heart disease and exercise test data were not significantly different between the two groups. Four independent predictors of mortality after renal transplantation were identified: age ⩾50 years (p = 0.002), LVESD ⩾3.5 cm (p = 0.002), maximal wall thickness ⩾1.4 cm (p = 0.014) and mitral annular calcification (p = 0.036). The 5‐year survival estimates for 0, 1, 2 and 3 prognostic factors were 96%, 86%, 69% and 38%, respectively. No patient had four prognostic factors. In patients ⩾50 years, the 5‐year survival estimates for 0, 1 and 2 additional prognostic factors were 73%, 45% and 18%, respectively.
Conclusion
In addition to selection according to current guidelines, age and three conventional echocardiography parameters may further improve risk stratification before renal transplantation.
Cardiovascular disease is the main cause of death in patients with end‐stage renal disease.1,2 In the first 5 years after renal transplantation, half of all deaths are cardiac,3 often in the presence of a functional graft.4 Although improved survival of renal transplant recipients compared with patients undergoing dialysis has been shown,5 cardiovascular mortality remains twice that of the general population.4 These patients are therefore carefully screened to identify and treat cardiovascular risk factors and disease according to current guidelines.6 Most studies have focused on coronary artery disease given the association with poor mortality in end‐stage renal disease, and guidelines exist on how this should be evaluated.6,7 Although several parameters defined by echocardiography have been shown to predict poor outcome in end‐stage renal disease,8,9 only limited data on echocardiographic prognostic factors in renal transplant recipients are available. Consequently, current guidelines do not include echocardiography parameters for patient selection before renal transplantation. Owing to the limited number of kidneys available for transplantation, optimal selection of potential candidates is crucial. The aim of this study was to identify patients with high mortality after renal transplantation.
Materials and methods
This study had approval from the local ethical committee. The study was performed in accordance with the Declaration of Helsinki.
Population
In this prospective observational study, we evaluated 219 consecutive patients who underwent renal transplantation between 1996 and 2001. Patients with severe and inoperable coronary artery disease or severely impaired left ventricular ejection fraction (LVEF) <25%, severe lung disease (forced expiratory volume in 1 s <1), body mass index >35 kg/m2 or weight <40 kg, HIV positive, active immunological disease, sepsis, severe peripheral vascular disease and those in remission from cancer treatment <5 years were not included in the transplant list. These criteria are in accordance with European Best Practice Guidelines.6 Clinical, haematological and biochemical parameters were recorded before renal transplantation at the time of echocardiography (mean (standard deviation (SD)) time from echo to transplantation 5.1 (2.2) months). A total of 16 (7%) patients had inadequate echocardiographic image quality and were excluded. Follow‐up started from the date of transplantation. Survival data were collected at clinic visits and by direct patient communication. The cause of death was identified from postmortem examination in 13 cases. Drugs were prescribed at the liberty of the team during follow‐up.
Assessment of coronary artery disease
The probability of coronary artery disease was assessed in all patients on clinical grounds, and subsequent screening investigations were determined according to three levels of risk.7 Low‐risk patients were asymptomatic, <40 years of age with no cardiovascular risk factors and had no assessment for coronary disease (n = 20). High‐risk patients included patients with diabetes and a history of ischaemic heart disease. The other patients were considered to be at intermediate risk and had either exercise testing according to the Bruce protocol or adenosine thallium scan. All high‐risk patients and those at intermediate risk who had inconclusive or positive stress tests underwent coronary angiography (n = 45, 22%). Patients with relevant coronary disease were revascularised before transplantation (n = 13).
Assessment of peripheral vascular disease
All patients were screened for peripheral vascular disease using clinical evaluation and lower limb duplex ultrasonography. Patients with >50% artery stenosis underwent angiography and were treated accordingly with surgery or angioplasty, if symptomatic (n = 5).
Echocardiography
Echocardiography examination was undertaken using a GE Vingmed System 5 machine (Horten, Norway). Owing to the changes in left ventricular volumes that occur between dialysis sessions, echo examinations were performed 15–20 h after a session, when the extracellular fluid volume is comparable to normal subjects.10,11 Two‐dimensional measurements were performed as recommended by the American Society of Echocardiography.12 LVEF was determined by modified biplane Simpson's rule. Measurements were averaged over three cardiac cycles. Impaired left ventricular systolic function was defined as LVEF <50%. Left ventricular mass index was calculated according to Devereux and Reichek.13 Left ventricular hypertrophy was defined as left ventricular mass index ⩾125 g/m2 in men and ⩾110 g/m2 in women. Mitral annular calcification was defined as an echodense band visualised throughout systole and diastole, distinguishable from the posterior mitral valve leaflet, and located anterior and parallel to the posterior left ventricular wall on M‐mode recordings.14 Transmitral inflow was recorded using pulsed‐wave Doppler recordings at the mitral valve leaflet tips in the apical four‐chamber view. Mitral (E/A) ratio and E deceleration time were measured. A restrictive filling pattern was defined as mitral early to late diastolic filling velocity (E/A) >2 ms and E deceleration time <150 ms.15
Statistical analysis
Continuous variables were expressed as mean (SD) and comparisons made using unpaired t test. Categorical variables were compared using χ2 analysis or Fisher's exact test. All statistical tests were two tailed, with a p value <0.05 indicating significance. We assessed cumulative risks by Kaplan–Meier analysis and prognostic factors with Cox univariate and multivariate analyses. The SPSS statistics package V.12.0 was used.
Inter‐observer and intra‐observer variability
Echocardiography images were collected and interpreted by six different sonographers over the study period. From random selection, 20 echo recordings were analysed by two independent observers and within a 3‐week period by one observer. Variability was expressed as the percentage difference between the two values divided by the mean of the two values. The parameters compared were left ventricular end‐systolic diameter (LVESD) and LVEF (calculated according to the modified biplane Simpson's technique). The interobserver variability was 10% and the intraobserver variability was 8% for LVESD. The interobserver variability was 12% and the intraobserver variability was 10% for LVEF.
Results
Population
Complete data were collected from 203 patients. Table 1 shows the baseline characteristics. In all, 123 (61%) patients received haemodialysis, 66 (32%) received peritoneal dialysis and 14 (7%) received no dialysis before renal transplantation. Left ventricular hypertrophy was present in 60% of the patients, as shown by echocardiography. Fifty four patients had poorly controlled hypertension as defined by blood pressure ⩾135/85 mm Hg. A total of 23 (11%) patients had left ventricular dilatation and 15 (7%) had impaired left ventricular systolic function. Among the 45 patients who underwent coronary angiography, 24 were normal, 14 had single‐vessel, 1 had two‐vessel and 6 had three‐vessel coronary artery disease. Six patients with single‐vessel disease had coronary angioplasty. One subsequently died from myocardial infarction 1.8 years after renal transplantation. All patients with two‐ and three‐vessel coronary disease (n = 7) had surgical revascularisation before transplantation and only one subsequently died from sepsis 2.3 years after renal transplantation.
Table 1 Population characteristics before renal transplantation.
| Mean (SD; range) age (years) | 47 (12; 19–72) |
| Men, n (%) | 141 (69.5) |
| Mean (SD; range) body mass index (kg/m2) | 24.3 (4.6; 16–36) |
| Smoker, n (%) | 42 (21) |
| Hypertension, n (%) | 175 (85) |
| Mean (SD; range) systolic BP (mm Hg) | 139 (17; 100–208) |
| Mean (SD; range) diastolic BP (mm Hg) | 76 (11; 50–110) |
| Mean (SD; range) cholesterol (mmol/l) | 4.88 (1.27; 2.1–12.1) |
| Mean (SD; range) triglyceride (mmol/l) | 0.43 (1.1; 0–8) |
| Diabetes, n (%) | 42 (21) |
| Family history of IHD, n (%) | 28 (14) |
| Medical history of IHD, n (%) | 8 (4) |
| Previous stroke, n (%) | 6 (3) |
| Peripheral vascular disease, n (%) | 18 (8.9) |
| Atrial fibrillation, n (%) | 5 (2.5) |
| Cardiac symptoms, n (%) | 87 (43) |
| Previous renal transplantation, n (%) | 30 (15) |
| Dialysis, n (%) | 123 (93) |
| Mean (SD; range) haemoglobin (g/dl) | 10.52 (1.7; 6–15) |
| Mean (SD; range) creatinine (μmol/l) | 745 (382; 195–1247) |
| Mean (SD; range) calcium (mmol/l) | 2.24 (0.25; 1.4–2.9) |
| Mean (SD; range) phosphate (mmol/l) | 1.7 (0.38; 0.93–3.16) |
| Mean (SD; range) albumin (g/l) | 35 (6.8; 17–54) |
| ECG abnormal, n (%) | 122 (60) |
| ECG left ventricular hypertrophy, n (%) | 87 (43) |
| Mean (SD; range) LVEDD (cm) | 4.9 (0.7; 3.4–6.5) |
| Mean (SD; range) LVESD (cm) | 3.1 (0.7; 1.9–4.9) |
| Mean (SD; range) LVEDV (cm3) | 99 (54; 25–259) |
| Mean (SD; range) LVESV (cm3) | 22 (18; 6–90) |
| Mean (SD; range) LVFS (%) | 39 (8; 20–60) |
| Mean (SD; range) LVEF (%) | 70 (12; 38–90) |
| Mean (SD; range) LA diameter (cm) | 4 (0.9; 2.4–5.1) |
| Mean (SD; range) maximal wall thickness (cm) | 1.2 (0.18; 0.8–1.8) |
| Mean (SD; range) LVMI | 124 (18; 85–155) |
| Mean (SD; range) LVMI men (n = 141) | 126 (19; 91–155) |
| Mean (SD; range) LVMI women (n = 62) | 119 (19; 85–146) |
| Mitral annular calcification, n (%) | 63 (31) |
BP, blood pressure; IHD, ischaemic heart disease; LA, left atrium; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVESD, left ventricular end‐systolic diameter; LVESV, left ventricular end‐systolic volume; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVMI, left ventricular mass index.
Survival
The mean (SD) follow‐up period was 3.6 (1.9) years (range 0.2–8.27 years). The survival status of all patients was known at the end of the study. From 203 patients, there were 22 deaths (10.8%), of which 12 were cardiovascular: myocardial infarction (n = 7), heart failure (n = 3) and ischaemic stroke (n = 2). Other causes of death included sepsis (n = 2), malignancy (n = 4) and multiorgan failure (n = 4). All but three of the deaths occurred despite the presence of a functional renal graft. The mean (SD) time to death after transplantation was 1.5 (1.6) years and the mean (SD) age at death was 57 (5) years. The 28‐day mortality was 1.5% (n = 3). The Kaplan–Meier 5 year estimates for all‐cause and cardiovascular mortality after renal transplantation were 24% and 15%, respectively. There was no death among the patients excluded for inadequate echocardiography image quality. Ten patients required hospital admission after renal transplantation for cardiovascular causes: myocardial infarction (n = 2), unstable angina (n = 2), heart failure (n = 5) and stroke (n = 1). In all, 9 patients (4.4%) had a myocardial infarction after renal transplantation. Seven of nine myocardial infarctions were lethal. This poor outcome after myocardial infarction is in keeping with previously published data.16
Differences between survivors and non‐survivors
Non‐survivors were significantly older, had increased left ventricular mass index, wall thickness, cavity dimensions, mitral annular calcification and lower ejection fraction (table 2). All variables remained significantly different when analysis was made between survivors and non‐survivors who died from cardiac causes. No significant differences were observed in drugs used, cardiovascular risk factors, exercise test results, haematological and biochemical variables, the proportion of patients undergoing dialysis, mode of dialysis or those with a previous transplant.
Table 2 Univariate comparison between survivors and non‐survivors.
| Parameter | Survivors | Non‐survivors | p Value* | Cardiac mortality | p Value** |
|---|---|---|---|---|---|
| Number (%) | 181 (89) | 22 (11) | 12 (6) | ||
| Mean (SD) age (years) | 46 (12) | 56 (8) | <0.001 | 57 (5) | 0.002 |
| Men, n | 124 | 17 | NS | 9 | NS |
| Mean (SD) body mass index (kg/m2) | 24.2 (4.5) | 25 (5.3) | NS | 22.8 (4) | NS |
| Smoker, n | 35 | 7 | NS | 5 | NS |
| Hypertension, n | 153 | 19 | NS | 10 | NS |
| Mean (SD) systolic BP (mm Hg) | 139 (17) | 144 (14) | NS | 150 (13) | 0.02 |
| Mean (SD) diastolic BP (mm Hg) | 76 (11) | 76 (11) | NS | 80 (9) | NS |
| Mean (SD) cholesterol (mmol/l) | 4.89 (1.23) | 4.81 (1.16) | NS | 5.23 (1.05) | NS |
| Mean (SD) triglycerides (mmol/l) | 0.42 (1.08) | 0.55 (1.37) | NS | 0.91 (1.78) | NS |
| Diabetes, n | 35 | 7 | NS | 3 | NS |
| Positive family history, n | 24 | 4 | NS | 2 | NS |
| History of IHD | 5 | 3 | 0.04 | 2 | 0.06 |
| Previous stroke, n | 5 | 1 | NS | 1 | NS |
| Peripheral vascular disease, n | 13 | 5 | 0.03 | 0 | 0.06 |
| Cardiac symptoms, n | 78 | 9 | NS | 5 | NS |
| Previous renal transplantion, n | 27 | 3 | NS | 2 | NS |
| Haemodialysis, n | 112 | 10 | NS | 4 | 0.02 |
| Mean (SD) haemoglobin (g/dl) | 10.56 (1.74) | 10.2 (1.3) | NS | 10.08 (1.46) | NS |
| Mean (SD) creatinine (μmol/l) | 731 (255) | 861 (310)( | NS | 677 (158) | NS |
| Mean (SD) calcium (mmol/l) | 2.25 (0.25) | 2.18 (0.23) | NS | 2.91 (0.14) | NS |
| Mean (SD) albumin (g/l) | 37 (8) | 32 (6) | NS | 33 (8) | NS |
| Mean (SD) phosphate (mmol/l) | 1.71 (0.39) | 1.68 (0.35) | NS | 1.67 (0.34) | NS |
| Mean (SD) LVESD (cm) | 3 (0.7) | 3.6 (0.7) | <0.001 | 3.7 (0.6) | 0.001 |
| Mean (SD) LVESV (cm3) | 21 (16) | 36 (21) | <0.001 | 39 (22) | 0.002 |
| Mean (SD) LVEDD (cm) | 4.8 (0.7) | 5.3 (0.8) | 0.002 | 5.5 (0.6) | <0.001 |
| Mean (SD) LVEDV (cm3) | 95 (51) | 136 (64) | 0.001 | 144 (74) | <0.001 |
| Mean (SD) LVFS (%) | 39 (8) | 32 (8) | <0.001 | 30 (7) | <0.001 |
| Mean (SD) LVEF (%) | 72 (11) | 60 (13) | <0.001 | 57 (14) | <0.001 |
| Mean (SD) LA (cm) | 4 (0.6) | 3.8 (0.6) | NS | 3.7 (0.7) | NS |
| Mean (SD) maximal wall thickness (cm) | 1.18 (0.17) | 1.29 (0.18) | 0.006 | 1.34 (0.19) | 0.007 |
| Mean (SD) LVMI (g/m2) | 122 (18) | 136 (11) | 0.001 | 135 (14) | 0.02 |
| Mean (SD) LVMI, men (n = 141) | 124 (17) | 137 (11) | 0.004 | 136 (10) | 0.02 |
| Mean (SD) LVMI, women (n = 62) | 117 (19) | 132 (10) | 0.009 | 130 (12) | 0.008 |
| Mitral restrictive filling pattern, n (%) | 19 (11) | 3 (14) | NS | 2 (16) | NS |
| Mitral annular calcification, n | 49 | 14 | 0.001 | 7 | NS |
BP, blood pressure; IHD, ischaemic heart disease; LA, left atrium; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVESD, left ventricular end‐systolic diameter; LVESV, left ventricular end‐systolic volume; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVMI, left ventricular mass index; NS, non‐significant.
*Comparison of survivors and non‐survivors.
**Comparison of survivors and non‐survivors who died from cardiac causes.
Independent predictors of mortality
Multivariate analysis by Cox regression was performed on all variables identified by univariate analysis to be significantly different between survivors and non‐survivors. Also included in the model were sex, blood pressure, mode and duration of dialysis. Four independent predictors of mortality were identified: age, LVESD, maximal left ventricular wall thickness and mitral annular calcification (table 3). The optimal cut‐off value for each of these parameters to predict mortality was determined from receiver operator characteristic curve analysis. Age ⩾50 years (area under curve = 0.75; p⩽0.001) predicted mortality with 74% sensitivity and 71% specificity. LVESD ⩾3.5 cm (area under curve = 0.76; p⩽0.001) predicted mortality with 72% sensitivity and 70% specificity. Maximal left ventricular wall thickness ⩾1.4 cm (area under curve = 0.73, p = 0.002) predicted mortality with 77% sensitivity and 72% specificity.
Table 3 Independent predictors of mortality by multivariate Cox regression analysis.
| p Value | Exp (β) | 95.0% CI | |
|---|---|---|---|
| Age | 0.003 | 1.06 | 1.021.11 |
| LVESD | 0.002 | 3.82 | 1.619.07 |
| Maximal wall thickness | 0.014 | 3.03 | 1.257.34 |
| Mitral annular calcification | 0.036 | 2.71 | 1.076.87 |
LVESD, left ventricular end‐systolic diameter.
Mortality according to the number of independent prognostic factors
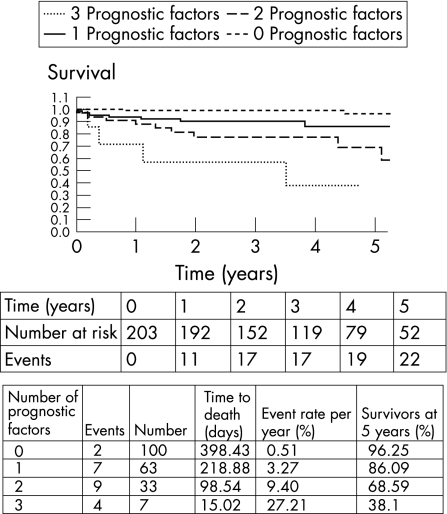
Kaplan–Meier survival analysis was performed to show the relationship between the number of independent predictors of mortality and outcome (fig 1). Table 4 shows a progressive decrease in survival with an increasing number of prognostic factors. The 5‐year survival estimates for 0, 1, 2 and 3 prognostic factors were 96%, 86%, 69% and 38%, respectively. None of the patients had four prognostic factors.
Figure 1 Survival according to the number of prognostic factors. A progressive decrease in survival with an increasing number of prognostic factors was observed.
Table 4 Prognosis index according to the number of independent predictive factors.
| Number of prognostic factors | p Value | Exp (β) | 95% CI |
|---|---|---|---|
| 1 | 0.036 | 5.38 | 1.11 to 26.01 |
| 2 | 0.001 | 13.92 | 2.98 to 65.06 |
| 3 | <0 | 31.56 | 5.65 to 176.34 |
None of the patients had four prognostic factors.
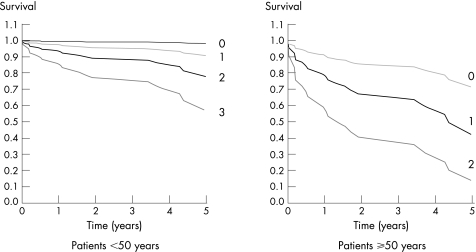
Patients were divided into two age groups: those ⩾50 and those <50 years. In each group, survival was assessed according to the presence of 1, 2 or 3 prognostic factors (fig 2). For patients <50 years, the 5‐year survival estimates for 1, 2 and 3 prognostic factors were 92%, 80% and 59%, respectively. For those >50 years, the corresponding 5‐year survival estimates were 73%, 45% and 18%, respectively.
Figure 2 Survival according to age and the number of prognostic factors. Patients ⩾50 years with two additional prognostic factors had a poor 5‐year survival rate after renal transplantation.
Discussion
Renal transplantation and dialysis are costly treatments for a growing proportion of the population. Efforts to reduce the high cardiovascular mortality and morbidity associated with renal replacement therapy are, to this point, limited to coronary revascularisation, changes in immunomodulators and use of lipid‐lowering agents. Despite these efforts, most of the transplant‐related deaths continue to be from cardiovascular disease. There remains the need to identify patients at highest risk to make a difference with current effective strategies. This study has shown that age and three parameters derived from conventional echocardiography can identify patients at increased mortality after renal transplantation, despite patient selection according to current guidelines. The results support previous studies suggesting that preoperative echocardiography parameters predict survival after renal transplantation.17,18 Unique to this study is the multivariate analysis based on age and the survival curves based on the number of prognostic factors. Those with no echo prognostic factors had good outcome irrespective of age. Patients ⩾50 years who had two additional prognostic factors had poor 5‐year survival after renal transplantation. The indication for renal transplantation has to be carefully evaluated in these patients and other comorbidities (stroke, lung disease, nutritional status) must be considered.
In this study, total and cardiac mortality after renal transplantation were 10.8% and 6%, respectively. Cardiovascular disease was the leading cause of death despite the use of a screening policy advocated by current guidelines.6 The incidence of cardiac death after renal transplantation was lower than that of previous studies. McGregor et al18 studied 141 renal transplant recipients between 1988 and 1990. Total and cardiac mortality were 24.1% and 15.6%, respectively. Ojo et al4 examined the records of 18 482 patients who received a transplant between 1988 and 1997. Total and cardiac mortality were 38% and 14%, respectively. The differences are probably due to different criteria for accepting patients into transplant waiting lists and the more aggressive cardiac screening guidelines followed in this study. Greater use of β‐blockers, ACE inhibitors and revascularisation may also have played a role.
Conventional cardiovascular risk factors and cardiac symptoms were not associated with an adverse outcome after transplantation, in keeping with previous reports.19 Given that previous studies have suggested adverse outcome in diabetes,20 the non‐significant difference in the proportion of patients with diabetes between survivors and non‐survivors seems surprising. The reasons for this are perhaps related to the more aggressive screening policy adopted for patients with diabetes in that they had angiography. Patients with diabetes were therefore more likely to be revascularised with better long‐term outcome,20 and those with severe coronary artery disease not amenable to treatment were not transplanted and thus not in this study. The latter may account for the small sample size of patients with diabetes with end‐stage renal disease (21%) in our population. Our study also suggested that a previous renal transplant was not associated with increased mortality. However, the patients recruited in this study were by definition survivors, and no data were collected related to the number of non‐survivors after a previous renal transplant. Therefore, this conclusion should be interpreted with caution.
Changes in left ventricular mass, size and function are common in patients with end‐stage renal disease and predict poor outcome in the dialysis population.21,22,23 Weinrauch et al17 showed that preoperative increased end‐systolic diameter and reduced velocity of circumferential fibre shortening predicted mortality in 47 patients with diabetes who underwent renal transplants. McGregor et al18 showed that age, left ventricular dilatation and impaired systolic function were markers of mortality in 141 patients evaluated before renal transplantation. Our results support both these studies. Preoperative left ventricular hypertrophy, left ventricular dilatation and reduced systolic function all predicted mortality after renal transplantation. This is despite studies suggesting an improvement of these parameters after renal transplantation.24,25 McGregor et al26 showed that an improvement of preoperative echoabnormalities after renal transplantation did not confer survival benefits.26 In our study, the echocardiography differences between survivors and non‐survivors were not explained by differences in drugs received, haemoglobin, albumin, body mass index or blood pressure. A restrictive filling pattern did not predict mortality in our patients. Reasons include the fact that this parameter is load dependent and predicts mortality predominantly in patients with heart failure.27
Left ventricular cavity dimensions and systolic function were normal in survivors and non‐survivors. However, patients with left ventricular hypertrophy often have enhanced systolic function, with ejection fraction >75% and smaller left ventricular cavity sizes.28 Therefore, a LVEF of 60% seen in non‐survivors (compared with 70% in survivors) represents a reduction for this population. LVESD is less preload‐dependent than ejection fraction and end‐diastolic diameter. This may explain why this parameter was most predictive of mortality in this population.
In all, 31% of patients had mitral annular calcification in keeping with previous studies showing extensive calcification in end‐ stage renal disease.29 This parameter was a marker of mortality in end‐stage renal disease. The results are consistent with studies in patients without renal disease, but differ from those of Shurmur et al,30 who concluded that there was an association with conduction defects but not with mortality in 66 patients undergoing dialysis with 12‐month follow‐up.30 Reasons for this difference include the larger sample size and longer follow‐up period of this study.
Four independent predictors of mortality after renal transplantation were identified: age ⩾50 years, LVESD ⩾3.5 cm, maximal wall thickness ⩾1.4 cm and mitral annular calcification. These factors were combined to produce a predictive model. Patients ⩾50 years with two additional prognostic factors had only 18% survival at 5 years, despite current transplant guidelines. Apart from age, the other independent prognostic variables identified in this study may well be surrogate end points for coronary artery disease and the duration and severity of hypertension rather than directly causative of mortality. Nevertheless, these parameters may be useful in identifying patients at highest risk who require further investigation and treatment before renal transplantation. Such high‐risk patients should be further investigated with coronary angiography given the variable results for myocardial perfusion imaging and exercise testing in this population. The recent results for dobutamine stress echocardiography have been more promising.31 As yet, there is no clear evidence that preoperative medical or surgical intervention of high‐risk patients with cardiac disease will result in improved survival after renal transplantation. Nevertheless, most transplant units advocate this strategy on the basis of studies in the general population and smaller observational studies in patients with end‐stage renal disease. The prognostic scoring system proposed by this study should be confirmed prospectively in larger populations before its routine clinical use can be recommended for the cardiac screening of potential renal transplant recipients.
Study limitations
Coronary angiography was only performed in high‐risk patients with cardiac disease, in keeping with UK Transplant guidelines during this study period.6 Given the limitations of non‐invasive screening tests, severe coronary disease may have been missed in our population. Only a single blood pressure recording was made in this study. Thus, long‐term effects of inadequately controlled hypertension cannot be inferred. The premise that cardiac dimensions and function can predict non‐cardiac mortality was not adequately considered in this study. To do this, more non‐cardiovascular data would be required.
Conclusion
Age and three prognostic factors derived from conventional echocardiography can identify patients at increased mortality after renal transplantation, despite selection according to current guidelines. A model based on a combination of these factors was a better predictor of outcome than any single parameter.
Abbreviations
LVEF - left ventricular ejection fraction
LVESD - left ventricular end‐systolic diameter
Footnotes
Competing interests: None.
References
- 1.Raine A E, Margreiter R, Brunner F P.et al Report on management of renal failure in Europe, XXII, 1991. Nephrol Dial Transpl 199277–35. [PubMed] [Google Scholar]
- 2.United States Renal Data System Incidence and prevalence of ESRD.Am J Kidney Dis 199832S38–S49. [DOI] [PubMed] [Google Scholar]
- 3.Lindholm A, Albrechtsen D, Frodin L.et al Ischemic heart disease—major cause of death and graft loss after renal transplantation in Scandinavia. Transplantation 199560451–457. [DOI] [PubMed] [Google Scholar]
- 4.Ojo A O, Hanson J A, Wolfe R A.et al Long‐term survival in renal transplant recipients with graft function. Kidney Int 200057307–313. [DOI] [PubMed] [Google Scholar]
- 5.Herzog C A, Ma J Z, Collins A J. Long‐term survival of renal transplant recipients in the United States after acute myocardial infarction. Am J Kidney Dis 200036145–152. [DOI] [PubMed] [Google Scholar]
- 6.EPBG (European Expert Group on Renal Transplantation; European Renal Association; European Society for Organ Transplantation European Best Practice Guidelines for Renal Transplantation (part 1). Nephrol Dial Transpl 200015(Suppl 7)1–85. [Google Scholar]
- 7.de Lemos J A, Hillis L D. Diagnosis and management of coronary artery disease in patients with end‐stage renal disease on hemodialysis. J Am Soc Nephrol 199672044–2054. [DOI] [PubMed] [Google Scholar]
- 8.Foley R N, Parfrey P S, Harnett J D.et al The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 199552024–2031. [DOI] [PubMed] [Google Scholar]
- 9.Foley R N, Parfrey P S, Harnett J D.et al Clinical and echocardiographic disease in patients starting end‐stage renal disease therapy. Kidney Int 199547186–192. [DOI] [PubMed] [Google Scholar]
- 10.Harnett J D, Murphy B, Collingwood P.et al The reliability and validity of echocardiographic measurement of left ventricular mass index in hemodialysis patients. Nephron 199365212–214. [DOI] [PubMed] [Google Scholar]
- 11.London G M, Fabiani F, Marchais S J.et al Uremic cardiomyopathy: an inadequate left ventricular hypertrophy. Kidney Int 198731973–980. [DOI] [PubMed] [Google Scholar]
- 12.Schiller N B, Shah P M, Crawford M.et al Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 13.Devereux R B, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 197755613–618. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin E J, Plehn J F, D'Agostino R B.et al Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med 1992327374–379. [DOI] [PubMed] [Google Scholar]
- 15.Appleton C P, Hatle L K, Popp R L. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 198812426–440. [DOI] [PubMed] [Google Scholar]
- 16.Herzog C A, Ma J Z, Collins A J. Poor long‐term survival after acute myocardial infarction among patients on long‐term dialysis. N Engl J Med 1998339799–805. [DOI] [PubMed] [Google Scholar]
- 17.Weinrauch L A, D'Elia J A, Monaco A P.et al Preoperative evaluation for diabetic renal transplantation: impact of clinical, laboratory, and echocardiographic parameters on patient and allograft survival. Am J Med 19929319–28. [DOI] [PubMed] [Google Scholar]
- 18.McGregor E, Jardine A G, Murray L S.et al Pre‐operative echocardiographic abnormalities and adverse outcome following renal transplantation. Nephrol Dial Transpl 1998131499–1505. [DOI] [PubMed] [Google Scholar]
- 19.Ma K W, Greene E L, Raij L. Cardiovascular risk factors in chronic renal failure and hemodialysis populations. Am J Kidney Dis 199219505–513. [DOI] [PubMed] [Google Scholar]
- 20.Manske C L, Wang Y, Rector T.et al Coronary revascularisation in insulin‐dependent diabetic patients with chronic renal failure. Lancet 1992340998–1002. [DOI] [PubMed] [Google Scholar]
- 21.Weinrauch L A, D'Elia J A, Gleason R E.et al Usefulness of left ventricular size and function in predicting survival in chronic dialysis patients with diabetes mellitus. Am J Cardiol 199270300–303. [DOI] [PubMed] [Google Scholar]
- 22.Parfrey P S, Foley R N, Harnett J D.et al Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transpl 1996111277–1285. [PubMed] [Google Scholar]
- 23.Zoccali C, Benedetto F A, Mallamaci F.et al Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol 2004151029–1037. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira S R, Moises V A, Tavares A.et al Cardiovascular effects of successful renal transplantation: a 1‐year sequential study of left ventricular morphology and function, and 24‐hour blood pressure profile. Transplantation 2002741580–1587. [DOI] [PubMed] [Google Scholar]
- 25.Wali R K, Wang G S, Gottlieb S S.et al Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end‐stage renal disease. J Am Coll Cardiol 2005451051–1060. [DOI] [PubMed] [Google Scholar]
- 26.McGregor E, Stewart G A, Rodger C.et al Early echocardiographic changes and survival following renal transplantation. Nephrol Dial Transpl 20001593–98. [DOI] [PubMed] [Google Scholar]
- 27.Pozzoli M, Traversi E, Cioffi G.et al Loading manipulations improve the prognostic value of Doppler evaluation of mitral flow in patients with chronic heart failure. Circulation 1997951222–1230. [DOI] [PubMed] [Google Scholar]
- 28.Reichek N. Echocardiographic assessment of left ventricular structure and function in hypertension. Methodology. Am J Med 19837519–25. [DOI] [PubMed] [Google Scholar]
- 29.Goodman W G, Goldin J, Kuizon B D.et al Coronary‐artery calcification in young adults with end‐stage renal disease who are undergoing dialysis. N Engl J Med 20003421478–1483. [DOI] [PubMed] [Google Scholar]
- 30.Shurmur S W, D'Elia J A, Gleason R E.et al Cardiac conduction defects associated with aortic and mitral valve calcification in dialysis patients. Ren Fail 199012103–107. [DOI] [PubMed] [Google Scholar]
- 31.Sharma R, Pellerin D, Gaze D C.et al Dobutamine stress echocardiography and the resting but not exercise electrocardiograph predict severe coronary artery disease in renal transplant candidates. Nephrol Dial Transpl 2005202207–2214. [DOI] [PubMed] [Google Scholar]