Abstract
Objective
Despite their clinical introduction 10 years ago, no human series on the healing response to Amplatzer and Starflex devices in humans have been reported yet. We sought to investigate the biocompatibility of Amplatzer and Cardioseal/Starflex septal occluder devices in humans and compare the findings to results in experimental animals.
Methods
The healing response of Amplatzer and Cardioseal/Starflex septal occluder devices in humans (n = 12, follow‐up periods from 5 days to 4 years) and in experimental animals (n = 32, follow‐up periods from 4 days to 1 year) was studied using a uniform work up protocol. Histological sections of paraffin‐wax‐embedded or methacrylate‐embedded specimen and scanning electron microscopy were used for biocompatibility screening.
Results
Neoendothelialisation of all examined devices was complete after 3 months in vivo. Protruding metal frame parts, like screw threads and spring arms, were covered last. The initial deposition of fibrin and blood cells on the polyester fabric was subsequently organised by ingrown fibroblastic cells. Loosely arranged and poorly vascularised young granulation tissue was transformed time‐dependently into quiescent fibre‐rich connective repair tissue poor of cellular and capillary vessel components. Consistently, a mild chronic inflammatory response directed against textile fibres of both types of implants characterised by lymphocytic infiltration and multinucleated foreign body giant cells was observed equally in human and animal explants.
Conclusions
Systematic biocompatibility screening in a series of explanted human septal occluder devices showed results corresponding to findings in animal studies with regard to neoendothelialisation, cellular organisation of initial thrombus and persisting immune response.
Over the past decade, the interventional closure of amenable atrial septal defects (ASD) has become the treatment of choice in most centres worldwide. Yet, very little histopathological‐based data on the biocompatibility of ASD closure devices in humans is currently available.1 Information on the biocompatibility of the various closure devices is primarily derived for series in experimental animals. These animal experiments, most often performed as safety and efficacy studies for clearance and (premarket) approval, however, have certain limitations. Follow‐up periods in vivo after device implantation in these studies usually range between 3 months and 2 years because of the necessity to obtain timely results from experiments which are highly demanding with regard to time, costs and effort.2,3,4,5,6,7,8 This relatively short follow‐up period with regard to local tissue response and biocompatibility contrasts with the expected life‐long implant persistence of some 70–80 years in young children.
Additionally, animals in experimental series have artificially created defects as opposed to congenital defects. This difference could potentially alter the healing and immune response after device implantation.
The aim of this comparative study was to elucidate whether data on biocompatibility from histopathological work up may be reliably transferred from animal studies to expected tissue reactions in humans. We, therefore, compared the biocompatibility of the Cardioseal/Starflex device and the Amplatzer ASD occluder device in a series of human explants with results from experimental animals of the same two device types using a uniform histopatholgical work up protocol.
Material and Methods
Animal experiments
ASDs were created in young female black‐headed mutton sheep (n = 32, mean (SD) body weight 32 (5) kg) by transseptal puncture and subsequent balloon dilatation of the interatrial septum2,9 under fluoroscopy and intracardiac echo control. After allowing for healing of the created ASD edges for 2–3 weeks, defect closure was performed using either Cardioseal/Starflex devices (NMT Medical, Boston, Massachusetts, USA, n = 24) or Amplatzer ASD occluder devices (AGA Medical, Golden Valley, Minnesota, USA, n = 8), respectively (table 1). Anaesthesia was introduced by xylazine 0.4 mg/kg intramuscularly and an intravenous bolus infusion of 10 mg/kg ketamine. Animals underwent endotracheal intubation and were mechanically ventilated with isofluorane and oxygen/room air. ECG, heart rate, respiratory rate, transcutaneous oxygen saturation, tidal volume and end‐tidal CO2 were monitored throughout the procedure. Before defect, occlusion heparin (200 units/kg) and antibiotics (flucloxacillin 1.5 g) were given intravenously and balloon sizing of the defect was performed (PTS sizing balloon catheter, NuMed, Cornwall, Ontario, Canada). Devices were deployed using standard techniques described previously10,11,12 under fluoroscopy and intracardiac echo control. Colour Doppler was used to assess potential residual shunting. Animals did not receive any anticoagulation during follow‐up. Table 1 details the follow‐up periods ranged from 4 days to 12 months. Before killing, each animal received an intravenous dose of heparin (400 units/kg body weight) to prevent postmortem clot formation on the device, followed by a lethal injection of pentobarbital.
Table 1 Sources and characterisation of specimens for histopathological work up.
| Animal experiments (n = 32) | |||||
|---|---|---|---|---|---|
| Implant | n | Implantation times | |||
| Amplatzer | 8 (2 groups) | 4 days, 1/3/6 months | |||
| Starflex | 24 (3 groups) | 7/14 days, 1/3/6/12 months | |||
| Human specimen (n = 12) | |||||
|---|---|---|---|---|---|
| Patient | Implant | Implantation time | Indication for explantation | ||
| 1 | Amplatzer ASD | 5 days | Malpositioning | ||
| 2 | Amplatzer VSD | 2 months | Valve incompetence | ||
| 3 | Cardioseal ASD | 5 months | Recurrent transient ischaemic attacks | ||
| 4 | Amplatzer ASD | 12 months | Malpositioning | ||
| 5 | Amplatzer ASD | 15 months | Residual shunt | ||
| 6 | Starflex ASD | 21 months | Deformation/residual shunt | ||
| 7 | Amplatzer ASD | 21 months | Malpositioning | ||
| 8 | Amplatzer ASD | 24 months | Residual shunt | ||
| 9 | Cardioseal ASD | 25 months | Residual shunt | ||
| 10 | Starflex ASD (2) | 31 months | Residual shunt | ||
| 11 | Cardioseal ASD | 36 months | Arm fracture, tissue proliferation atrial surface | ||
| 12 | Starflex ASD | 48 months | Recurrent neurology, tissue proliferation atrial surface | ||
ASD, atrial septal defect; VSD, ventricular septal defect.
All animals received humane care in compliance with the Guide for the care and use of laboratory animals published by the US National Institutes of Health (NIH Publication No 85–23, revised 1996). The study had been approved by the local governmental animal ethics committee.
Human explants
Human tissue specimens were collected during corrective surgery at various centres and send to us for histopathological work up. The implant durations of the ASD closure devices ranged from 5 days to 48 months. Table 1 summarises the reasons for device explantations.
Tissue preparation
Immediately after explantation, the tissue block containing the implant was dissected free with a minimum of surrounding tissue. After briefly flushing with saline, macroscopic evaluation and documentation was accomplished. Parts of the specimen arranged for histology and immunohistochemistry were fixed in formalin (buffered 4%). For scanning electron microscopy, usually one part of the specimen was put in glutaraldehyde (2.5%).
Embedding, sectioning and histology
After fixation, the main part of the tissue block with the device was embedded in the resins hydroxyethylmethacrylate (Technovit 7200 or Technovit 8100, Kultzer & Co, Wehrheim, Germany) or methylmethacrylate (Technovit 9100, Kultzer & Co, Wehrheim, Germany). After hardening, the resin blocks were subsequently sectioned in slices of 0.8 mm using a diamond band saw (300 CP, Exakt GmbH, Norderstedt, Germany). These slices were grinded down to 5–30 μm with a horizontal rotatory grinder and polisher (400 CS, Exakt, Norderstedt, Germany).
Parts not containing metal (eg, structures neighbouring the site of the implant, or parts only containing polyester fabric) were dehydrated and put in paraffin wax according to routine protocols. Staining of resin embedded specimen was performed with toluidine blue or Richardson blue. Paraffin‐wax‐embedded specimens were stained with haematoxylin and eosin.
Scanning electron microscopy
Whenever possible, one part of the specimens was prepared for scanning electron microscopy (SEM) and placed in glutaraldehyde (2.5%) for fixation. Tissue samples were dried by means of critical‐point‐drying and then sputtered with gold. A scanning electron microscope (Zeiss DSM 940, Oberkochen, Germany) was used for examination.
Results
Gross pathology
On gross inspection all devices but one were macroscopically intact. One arm fracture was observed in a 28 mm Cardioseal ASD occluder 36 months after implantation (patient 11). With one exception, no significant compromise of blood flow at the pulmonary or systemic veins or at the heart valves was seen in any of the patients (transthoracic or transoesophageal echocardiography) or animals (intracardial echocardiography and fluoroscopy). In patient 2, an Amplatzer device was removed surgically 2 months after the closure of a perimembranous ventricular septal defect because of progressive aortic valve incompetence.
Blood–implant interface
Devices explanted after only a very short follow‐up period of 4–7 days in vivo showed little yellow‐reddish, friable material on the device surfaces (fig 1A–C). These deposits were mainly observed in the periphery of the devices neighbouring the defect rims and around protruding parts of the metal framework like the screw thread of the Amplatzer device and along the spring arms of Cardioseal/Starflex devices. Histopathological work up of these early explants from both humans (patient 1) and experimental animals showed that the deposited material around the polyester meshwork consisted of fibrin, condensed plasma proteins and blood cells (fig 3A,B).
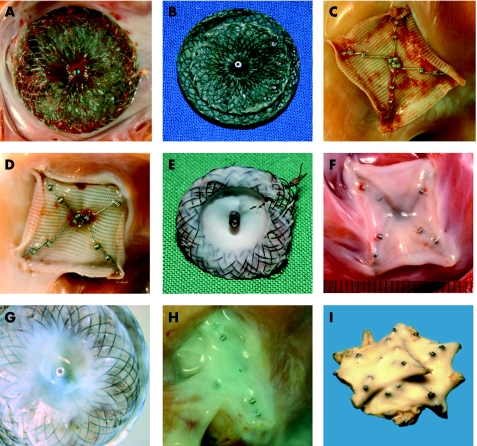
Figure 1 Gross pathology. Macroscopic aspects of occluder devices with different implantation times. Superficial coverage with fibrin in an Amplatzer occluder (animal, fig 1A) 4 days after implantation; in an Amplatzer occluder (human, fig 1B) 5 days after implantation, and in a Starflex occluder (animal, fig 1C) 7 days after implantation. Thin cellular coverage with a smooth shining surface in a Starflex occluder (animal, fig 1D) 1 month, in an Amplatzer ventricular septal defect occluder (human, fig 1E) 2 months, and in a Starflex occluder (animal, fig 1F) 3 months after implantation. Advanced cellular coverage in an Amplatzer occluder (human, fig 1G) and a Starflex occluder (animal, fig 1H) both 12 months after implantation, and in two Starflex devices (human, fig 1I) 31 months after implantation for multiple ASDs.
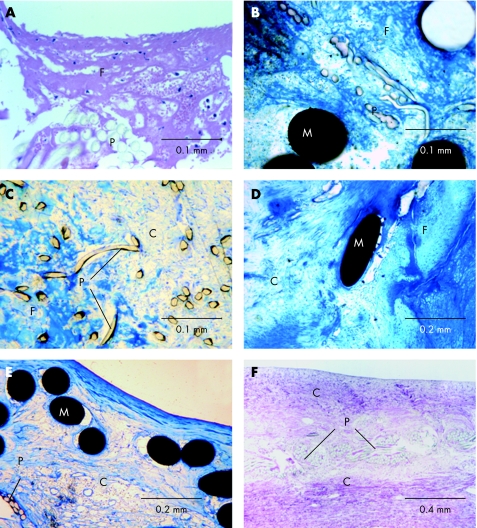
Figure 3 Blood–implant interface and tissue formation within the implant. Histological images showing progress of tissue organisation after device implantation. Superficial fibrin condensation with incorporated blood cells in a Starflex occluder (animal, implantation time 7 days, fig 3A) and in an Amplatzer occluder (human, implantation time 5 days, fig 3B). Partial cellular organisation with remaining islets of thrombotic material in the inner part of the device in an Amplatzer occluder (human, implantation time 2 months, fig 3C) and in an Amplatzer occluder (animal, implantation time 3 months, fig 3D). Complete cellular organisation in a Starflex occluder (animal, implantation time 12 months, fig 3E) and in an Amplatzer occluder (human, implantation time 24 months, fig 3F). C, cells; F, fibrin/thrombus; M, metal struts; P, polyester fibres.
Animals with an implantation time of 30 days and a human explant after 2 months in vivo (patient 2) showed an almost complete coverage except for the central pin and metal arms (Cardioseal/Starflex) or central struts and screw thread (Amplatzer) by a shiny, glistening surface layer through which the polyester fabric could be seen (fig 1D,E). Early neoendothelial coverage was observed to occur equally distributed over the entire surface of the device without a preference of the device periphery bordering the defect edges. SEM disclosed incomplete neoendothelialisation of the protruding parts of the metal framework after 30 days in vivo. Figure 2A shows an uncovered distal spring joint (“wrist joint”) and arm element of a Starflex device. At the same time, the polyester fabric of the device is already continuously covered by neoendothelium. After implantation times of 90, 180 or 360 days in animals or 5–15 months in humans respectively (patients 3–5), an increasing amount of whitish, dull tissue developed underneath a complete glistening surface layer with the fabric and (parts of) the frame remaining visible through this tissue (fig 1F–H). Methacrylate embedded stains exhibited not only endothelial coverage of the device fabric, but also the metal framework (fig 3E,F). In line with this, SEM performed on specimens with a follow‐up period of ⩾90 days in vivo showed complete neoendothelial coverage of the protruding metal framework, both in devices from experimental animals (fig 2B) as well as from humans (fig 2C, patient 3).
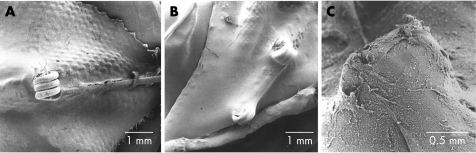
Figure 2 Scanning electron microscopy. Scanning electron microscopy images showing endothelial coverage with only prominent parts uncovered in a Starflex occluder 30 days after implantation (animal, fig 2A), and completed endothelialisation of arm joints after 3 months (animal, fig 2B) and 5 months (human, fig 2C).
In patients with implantation times of ⩾21 months, incorporation of the implants in whitish smooth tissue was thorough, with only straight parts of the metal frame (joint coils in Cardioseal/Starflex or screw threads in Amplatzer) shining through (fig 1I, patient 10). On careful examination, there was no evidence of superficial thrombus formation in any of the specimens evaluated for this study.
Cellular organisation within the implants
Immediately after implantation, fibrin condensation and accumulation of thrombotic material was shown within the implant in both types of device (fig 3A, animal, fig 3B, patient 1). Cellular organisation was shown to proceed in the initial months after implantation with ingrowth of fibromuscular cells and the formation of loosely arranged and poorly vascularised young granulation tissue (fig 3C, patient 2; fig 3D, animal). At this stage and all the later stages of the healing process, a considerably thicker layer of fiboblastoid granulation tissue was observed in the device periphery as compared with the central parts of the fabric, indicating migration of fibroblastic cells (with subsequent synthesis of fibres) from the defect edge on to the device surface. Within the second half of the first year, transformation of the early thrombus material was completed and the histology was dominated by connective tissue containing capillaries and small vessels both in animals and in humans (fig 3E, patient 8, fig 3F, animal). Later stages of follow‐up showed a relatively quiescent picture of the connective repair tissue which seemed fibre‐rich and poor of cellular and capillary vessel components.
Implant‐related inflammatory reactions
With exception of the implants with the shortest implantation time (5 days in patient 1; 4–7 days for animal groups), many multinucleated foreign body giant cells were found neighbouring the polyester fibres in both types of occluder devices. Both, humans and experimental animals corresponded in respect to amount and local distribution of this cellular foreign body reaction (fig 4A,B). Additionally, circumscribed mild lymphocytic infiltrations were observed with a loose distribution within the implants, but constantly related locally to the polyester fibres (fig 4C,D). These two types of mild inflammatory reactions against the device scaffold were of chronic nature: with some interindividual variation, the presence of multinucleated giant cells and lymphocytic infiltrates persisted and was observed also in the longest‐term implants (1‐year animal group, patient 12 after 4 years) without a tendency to diminish in its extent.
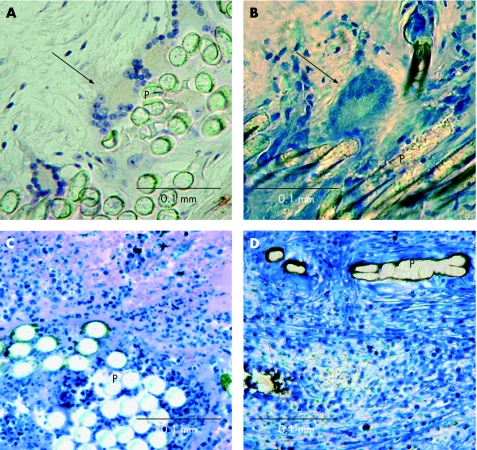
Figure 4 Inflammatory reactions. Histological images of inflammatory reactions directed against foreign material of septal closure devices. Foreign body giant cells (arrows) at the polyester fibres (P) within a Starflex occluder (animal, implantation time 3 months, fig 4A), and within an Amplatzer occluder (human, implantation time 2 months, fig 4B). Lymphocytic infiltrations in a Starflex occluder (animal, implantation time 3 months, fig 4C) and in an Amplatzer occluder (human, implantation time 24 months, fig 4D).
Discussion
This study contains the largest published histopathology work up series on human septal occluders with the widest range of follow‐up periods and is, to the best of our knowledge, the first that directly and systematically compares the explant findings between human and experimental animal series of two of the most common currently used septal occluders, the Amplatzer and Cardioseal/Starflex devices.
Hitherto, the biocompatibility and histopathology of the healing response of the various septal closure devices has virtually only been investigated in preclinical animal studies performed for regulatory (premarket) device approval.2,3,4,5,6,7,8 Besides individual case reports,13,14 there is only one published series in humans describing the healing response to the historical clampshell device used for various defect closures performed during regulatory trials.1 This device was withdrawn from the market owing to a high rate of metal arm fractures15,16 and—following a redesign of the framework—replaced by the Cardioseal device in 1996 and its self‐centring modification, the Starflex device in 1998. Besides the inclusion of these newer double umbrella type devices, this study also contains the first published series of the histological healing response to Amplatzer devices in humans.
So far, no systematic comparison between the healing response in the experimental animal setting and the healing pattern in humans has been made. We deem this point noteworthy as not only the nature of the defects, but also the postinterventional treatment differs between the clinical and experimental situation. Artificial defects, created by trans‐septal puncture and subsequent balloon dilation, as used in the sheep model in this study, result from a fresh wound in the septal wall. Even after allowing for healing of the defect edges (2–3 weeks in this study) this could potentially alter the healing response to devices implanted shortly thereafter. Furthermore, although it is common clinical practice to prescribe aspirin alone or in combination with clopidogrel for a postinterventional period of 3–6 months, animals analysed within this and other studies received no anticoagulation at all after device placement.2,3,4,5,6,7,8 Not only is the daily application of the above mentioned treatment troublesome and costly in the long‐term experimental setting, but also there is some evidence in the literature that aspirin fails to inhibit platelet aggregation in sheep.17
Residual shunting was the most common indication for device removal in our work up series of human septal occluders (8 of 12 devices). It has been argued that these specimens represent a “negative selection” with regard to the healing response.1 However, at the histological level we observed no significant difference in the healing process of devices that were removed for residual shunting as compared with those without leakage during follow‐up. This is further substantiated by the fact that we observed a healing response similar in quality and course in animals with effective shunt closure.
In detail, explants from both humans and studies in the animal model showed only deposits of condensed plasma proteins, such as fibrin, and blood cells on their surfaces after 1 week in vivo. This thin layer of fragile yet unorganised thrombotic material seals the porous polyester meshwork that the Amplatzer and the Cardioseal/Starflex devices have in common. In accordance with previous reports,1,3,4 neoendothelial coverage of most parts of the polyester fabric was observed within the first month after implantation. The fact that an endothelial lining was observed in central parts of the device no later than in the periphery may further substantiate the argument that blood‐borne progenitor cells play an important role in the neoendothelialisation of the implant.18,19
Protruding parts of the metal framework like the screw thread of the Amplatzer device and the spring arms of Cardioseal/Starflex devices were the last parts of the septal occluders to endothelialise as shown by SEM and methacrylate resin embedding (after 3–5 months in vivo). This result is of clinical relevance. Most of the device‐related thrombi were detected at these early stages during follow‐up20,21 and from the information available in the literature, the locations of the thrombus attachments coincide well with findings of our study.22,23,24 The corresponding time‐frame of the neoendothelialisation of the devices in humans and experimental animals substantiates the common clinical practice to provide antiplatelet treatment for 3–6 months after device placement.
An ongoing inflammatory reaction directed against textile fibres of both implants was observed with development of multinucleated foreign body giant cells. Additionally, mild lymphocytic infiltrates occurred without correlation to length of follow‐up in vivo or type of implant and were observed equally in both human and animal explants. A reaction directed against the metal framework of the two devices investigated (nitinol in Amplatzer and MP35n in Cardioseal/Starflex devices) was not noticed. Methacrylate resin embedding proved to be mandatory for this analysis as this work up technique is a prerequisite for the histological analysis of the metal–tissue interface.25,26 All specimens showed an intimate spatial relationship between the described cellular reactions and the synthetic polyester fibres. Concurring with previous literature reports, no decline of the inflammatory reactions with prolonged observation time was observed1,3 and long‐term specimens from our study clearly show a chronically persisting inflammatory response, both in animals and humans (fig 4D).
In summary, biocompatibility screening in a collection of human tissue specimens of two frequently used types of septal occluders—the Amplatzer and Cardioseal/Starflex devices—revealed results corresponding closely to findings in animal series. Based on this comparative study, we conclude that tissue reactions in experimental animals adequately reflect the healing response to septal occluder devices in humans with regard to neoendothelialisation, cellular organisation of initial thrombus, and a chronically persisting inflammatory response.
Footnotes
Competing interests: None declared.
References
- 1.Kreutzer J, Ryan C A, Gauvreau K.et al Healing response to the clamshell device for closure of intracardiac defects in humans. Catheter Cardiovasc Interv 200154101–111. [DOI] [PubMed] [Google Scholar]
- 2.Lock J E, Rome J J, Davis R.et al Transcatheter closure of atrial septal defects. Experimental studies. Circulation 1989791091–1099. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn M A, Latson L A, Cheatham J P.et al Biological response to Bard clamshell septal occluders in the canine heart. Circulation 1996931459–1463. [DOI] [PubMed] [Google Scholar]
- 4.Sharafuddin M J, Gu X, Titus J L.et al Transvenous closure of secundum atrial septal defects: preliminary results with a new self‐expanding nitinol prosthesis in a swine model. Circulation 1997952162–2168. [DOI] [PubMed] [Google Scholar]
- 5.Das G S, Voss G, Jarvis G.et al Experimental atrial septal defect closure with a new, transcatheter, self‐centering device. Circulation 1993881754–1764. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen A B, Schneider M, Baandrup U.et al Animal experimental implantation of an atrial septal defect occluder system. Heart 199880606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zahn E M, Wilson N, Cutright W.et al Development and testing of the helex septal occluder, a new expanded polytetrafluoroethylene atrial septal defect occlusion system. Circulation 2001104711–716. [DOI] [PubMed] [Google Scholar]
- 8.Sideris E B, Sideris S E, Fowlkes J P.et al Transvenous atrial septal‐defect occlusion in piglets with a buttoned double‐disk device. Circulation 199081312–318. [DOI] [PubMed] [Google Scholar]
- 9.Jux C, Bertram H, Wohlsein P.et al Experimental ASD closure using autologous cell‐seeded interventional closure devices. Cardiovasc Res 200253181–191. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorf G. StarFlex ASD closure: deployment, techniques, equipment. J Interv Cardiol 20011469–76. [DOI] [PubMed] [Google Scholar]
- 11.Waight D J, Koenig P R, Cao Q L.et al Transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: clinical experience and technical considerations. Curr Interv Cardiol Rep 2000270–77. [PubMed] [Google Scholar]
- 12.Masura J, Gavora P, Formanek A.et al Transcatheter closure of secundum atrial septal defects using the new self‐centering Amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn 199742388–393. [DOI] [PubMed] [Google Scholar]
- 13.Sigler M, Paul T, Grabitz R G. Biocompatibility screening in cardiovascular implants. Z Kardiol 200594383–391. [DOI] [PubMed] [Google Scholar]
- 14.Prewitt K C, Gaither N S, Farb A.et al Transient ischemic attacks after long‐term clamshell occluder implantation for closure of atrial septal defect. Am Heart J 19921241394–1397. [DOI] [PubMed] [Google Scholar]
- 15.Bridges N D, Hellenbrand W, Latson L.et al Transcatheter closure of patent foramen ovale after presumed paradoxical embolism. Circulation 1992861902–1908. [DOI] [PubMed] [Google Scholar]
- 16.Prieto L R, Foreman C K, Cheatham J P.et al Intermediate‐term outcome of transcatheter secundum atrial septal defect closure using the Bard clamshell septal umbrella. Am J Cardiol 1996781310–1312. [DOI] [PubMed] [Google Scholar]
- 17.Spanos H G. Aspirin fails to inhibit platelet aggregation in sheep. Thromb Res 199372175–182. [DOI] [PubMed] [Google Scholar]
- 18.Shi Q, Rafii S, Wu M H.et al Evidence for circulating bone marrow‐derived endothelial cells. Blood 199892362–367. [PubMed] [Google Scholar]
- 19.Rafii S, Meeus S, Dias S.et al Contribution of marrow‐derived progenitors to vascular and cardiac regeneration. Semin Cell Dev Biol 20021361–67. [DOI] [PubMed] [Google Scholar]
- 20.Krumsdorf U, Ostermayer S, Billinger K.et al Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1000 consecutive patients. J Am Coll Cardiol 200443302–309. [DOI] [PubMed] [Google Scholar]
- 21.Sherman J M, Hagler D J, Cetta F. Thrombosis after septal closure device placement: a review of the current literature. Catheter Cardiovasc Interv 200463486–489. [DOI] [PubMed] [Google Scholar]
- 22.Acar P, Aggoun Y, Abdel‐Massih T. Images in cardiology: thrombus after transcatheter closure of ASD with an Amplatzer septal occluder assessed by three dimensional echocardiographic reconstruction. Heart 20028852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willcoxson F E, Thomson J D, Gibbs J L. Successful treatment of left atrial disk thrombus on an Amplatzer atrial septal defect occluder with abciximab and heparin. Heart 200490e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chessa M, Carminati M, Butera G.et al Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 2002391061–1065. [DOI] [PubMed] [Google Scholar]
- 25.Sigler M, Handt S, Seghaye M C.et al Evaluation of in vivo biocompatibility of different devices for interventional closure of the patent ductus arteriosus in an animal model. Heart 200083570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik N, Gunn J, Holt C M.et al Intravascular stents: a new technique for tissue processing for histology, immunohistochemistry, and transmission electron microscopy. Heart 199880509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]