Abstract
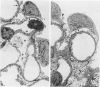
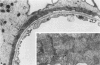
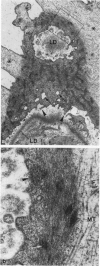
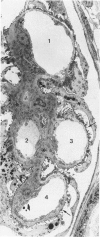
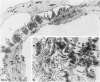
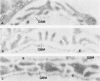
Foot process effacement represents the most characteristic change in podocyte phenotype under a great variety of experimental as well as human glomerulopathies. It consists in simplification up to a total disappearance of an interdigitating foot process pattern. Finally, podocytes affix to the glomerular basement membrane by outspread epithelial sheets. Structural and immunocytochemical techniques were applied to analyze the cytoskeletal changes associated with foot process effacement in Masugi nephritis. Three days after injection of the anti-glomerular-basement-membrane serum an interdigitating foot process pattern was almost fully lost; more than 90 percent of the outer glomerular capillary surface were covered by expanded sheets of podocyte epithelium that contain a highly organized cytoskeleton adhering to the basal cell membrane. Structurally, this cytoskeleton consists of an interwoven network of microfilaments with regularly distributed dense bodies, which obviously serve as cross-linkers within this network. Immunocytochemically, the expression of actin, alpha-actinin, and pp44 (a specific podocyte protein normally associated with the cytoskeleton of foot processes) were increased in this structure; alpha-actinin was especially prominent in the dense bodies. The results are consistent with the view that foot process effacement represents an adaptive change in cell shape including hypertrophy of the contractile apparatus, reinforcing the supportive role of podocytes. Several factors associated with increased distending forces to podocytes may underlie this phenotype change including loss of mesangial support, elevated glomerular pressures, and impairment of GBM substructure as well as of podocyte-GBM-contacts. Twenty-eight days after serum injection a remodeling of the foot process pattern was seen. It appears that this restitution depends on a preceding repair of mesangial support function to glomerular capillaries.
Full text
PDF







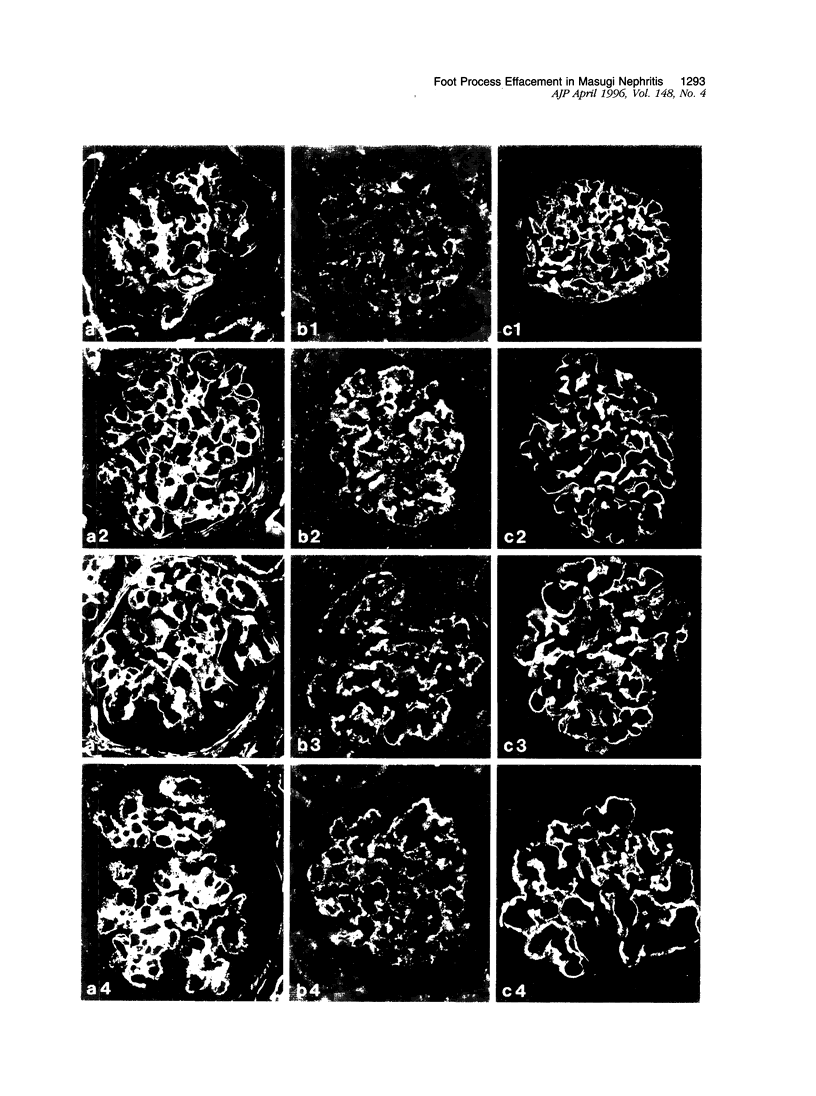



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. Integrin receptors in the glomerulus: potential role in glomerular injury. Am J Physiol. 1992 May;262(5 Pt 2):F697–F704. doi: 10.1152/ajprenal.1992.262.5.F697. [DOI] [PubMed] [Google Scholar]
- Andrews P. M. Cationized ferritin binding to anionic surfaces in normal and aminonucleoside nephrotic kidneys. Am J Anat. 1981 Oct;162(2):89–106. doi: 10.1002/aja.1001620202. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Wilson C. B. Acute effects of antiglomerular basement membrane antibody on the process of glomerular filtration in the rat. J Clin Invest. 1976 Oct;58(4):899–911. doi: 10.1172/JCI108543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandis A., Bianchi G., Reale E., Helmchen U., Kühn K. Age-dependent glomerulosclerosis and proteinuria occurring in rats of the Milan normotensive strain and not in rats of the Milan hypertensive strain. Lab Invest. 1986 Aug;55(2):234–243. [PubMed] [Google Scholar]
- Drenckhahn D., Franke R. P. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988 Nov;59(5):673–682. [PubMed] [Google Scholar]
- Drumond M. C., Kristal B., Myers B. D., Deen W. M. Structural basis for reduced glomerular filtration capacity in nephrotic humans. J Clin Invest. 1994 Sep;94(3):1187–1195. doi: 10.1172/JCI117435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin L. D., Hostetter T. H., Rennke H. G., Brenner B. M. Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest. 1984 May;73(5):1448–1461. doi: 10.1172/JCI111349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med. 1961 Nov 1;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., VERNIER R. L., GOOD R. A. An electron microscope study of the glomerulus in nephrosis, glomerulonephritis, and lupus erythematosus. J Exp Med. 1957 Nov 1;106(5):649–660. doi: 10.1084/jem.106.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj A. H., Morley A. R. Remnant kidney pathology after five-sixth nephrectomy in rat. II. Electron microscopy study. APMIS. 1993 Jan;101(1):83–90. doi: 10.1111/j.1699-0463.1993.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Fox J. E. Transmembrane signaling across the platelet integrin glycoprotein IIb-IIIa. Ann N Y Acad Sci. 1994 Apr 18;714:75–87. doi: 10.1111/j.1749-6632.1994.tb12032.x. [DOI] [PubMed] [Google Scholar]
- Grishman E., Churg J. Focal glomerular sclerosis in nephrotic patients: an electron microscopic study of glomerular podocytes. Kidney Int. 1975 Feb;7(2):111–122. doi: 10.1038/ki.1975.16. [DOI] [PubMed] [Google Scholar]
- Guasch A., Myers B. D. Determinants of glomerular hypofiltration in nephrotic patients with minimal change nephropathy. J Am Soc Nephrol. 1994 Feb;4(8):1571–1581. doi: 10.1681/ASN.V481571. [DOI] [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Kaissling B., Kriz W. Variability of intercellular spaces between macula densa cells: a transmission electron microscopic study in rabbits and rats. Kidney Int Suppl. 1982 Aug;12:S9–17. [PubMed] [Google Scholar]
- Kerjaschki D. Molecular pathogenesis of membranous nephropathy. Kidney Int. 1992 Apr;41(4):1090–1105. doi: 10.1038/ki.1992.166. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D. Polycation-induced dislocation of slit diaphragms and formation of cell junctions in rat kidney glomeruli: the effects of low temperature, divalent cations, colchicine, and cytochalasin B. Lab Invest. 1978 Nov;39(5):430–440. [PubMed] [Google Scholar]
- Kondo Y., Shigematsu H., Okabayashi A. Cellular aspects of rabbit Masugi nephritis. III. Mesangial changes. Lab Invest. 1976 Apr;34(4):363–371. [PubMed] [Google Scholar]
- Kretzler M., Koeppen-Hagemann I., Kriz W. Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomised-desoxycorticosterone hypertensive rat. Virchows Arch. 1994;425(2):181–193. doi: 10.1007/BF00230355. [DOI] [PubMed] [Google Scholar]
- Kriz W., Elger M., Mundel P., Lemley K. V. Structure-stabilizing forces in the glomerular tuft. J Am Soc Nephrol. 1995 Apr;5(10):1731–1739. doi: 10.1681/ASN.V5101731. [DOI] [PubMed] [Google Scholar]
- Kriz W., Hähnel B., Rösener S., Elger M. Long-term treatment of rats with FGF-2 results in focal segmental glomerulosclerosis. Kidney Int. 1995 Nov;48(5):1435–1450. doi: 10.1038/ki.1995.433. [DOI] [PubMed] [Google Scholar]
- Kühn K., Ryan G. B., Hein S. J., Galaske R. G., Karnovsky M. J. An ultrastructural study of the mechanisms of proteinuria in rat nephrotoxic nephritis. Lab Invest. 1977 Apr;36(4):375–387. [PubMed] [Google Scholar]
- Lemanski L. F., Paulson D. J., Hill C. S., Davis L. A., Riles L. C., Lim S. S. Immunoelectron microscopic localization of alpha-actinin on Lowicryl-embedded thin-sectioned tissues. J Histochem Cytochem. 1985 Jun;33(6):515–522. doi: 10.1177/33.6.3889138. [DOI] [PubMed] [Google Scholar]
- Lemley K. V., Elger M., Koeppen-Hagemann I., Kretzler M., Nagata M., Sakai T., Uiker S., Kriz W. The glomerular mesangium: capillary support function and its failure under experimental conditions. Clin Investig. 1992 Sep;70(9):843–856. doi: 10.1007/BF00180755. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Bennett C. M., Deen W. M., Glassock R. J., Knutson D., Daugharty T. M., Brenner B. M. Determinants of glomerular filtration in experimental glomerulonephritis in the rat. J Clin Invest. 1975 Feb;55(2):305–318. doi: 10.1172/JCI107934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina A., Davies D. J., Dillane P. C., Ryan G. B. Glomerular epithelial abnormalities associated with the onset of proteinuria in aminonucleoside nephrosis. Am J Pathol. 1987 Feb;126(2):220–229. [PMC free article] [PubMed] [Google Scholar]
- Mundel P., Gilbert P., Kriz W. Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. J Histochem Cytochem. 1991 Aug;39(8):1047–1056. doi: 10.1177/39.8.1856454. [DOI] [PubMed] [Google Scholar]
- O'Meara Y. M., Natori Y., Minto A. W., Goldstein D. J., Manning E. C., Salant D. J. Nephrotoxic antiserum identifies a beta 1-integrin on rat glomerular epithelial cells. Am J Physiol. 1992 Jun;262(6 Pt 2):F1083–F1091. doi: 10.1152/ajprenal.1992.262.6.F1083. [DOI] [PubMed] [Google Scholar]
- Olson J. L., Heptinstall R. H. Nonimmunologic mechanisms of glomerular injury. Lab Invest. 1988 Nov;59(5):564–578. [PubMed] [Google Scholar]
- Ryan G. B., Karnovsky M. J. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975 Oct;8(4):219–232. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- Sakai T., Kriz W. The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol (Berl) 1987;176(3):373–386. doi: 10.1007/BF00310191. [DOI] [PubMed] [Google Scholar]
- Seiler M. W., Rennke H. G., Venkatachalam M. A., Cotran R. S. Pathogenesis of polycation-induced alterations ("fusion") of glomerular epithelium. Lab Invest. 1977 Jan;36(1):48–61. [PubMed] [Google Scholar]
- Shimamura T., Morrison A. B. A progressive glomerulosclerosis occurring in partial five-sixths nephrectomized rats. Am J Pathol. 1975 Apr;79(1):95–106. [PMC free article] [PubMed] [Google Scholar]
- Shirato I., Sakai T., Fukui M., Tomino Y., Koide H. Widening of capillary neck and alteration of extracellular matrix ultrastructure in diabetic rat glomerulus as revealed by computer morphometry and improved tissue processing. Virchows Arch A Pathol Anat Histopathol. 1993;423(2):121–129. doi: 10.1007/BF01606586. [DOI] [PubMed] [Google Scholar]
- Velosa J. A., Donadio J. V., Jr, Holley K. E. Focal sclerosing glomerulonephropathy: a clinicopathologic study. Mayo Clin Proc. 1975 Mar;50(3):121–133. [PubMed] [Google Scholar]
- Whiteside C. I., Cameron R., Munk S., Levy J. Podocytic cytoskeletal disaggregation and basement-membrane detachment in puromycin aminonucleoside nephrosis. Am J Pathol. 1993 May;142(5):1641–1653. [PMC free article] [PubMed] [Google Scholar]