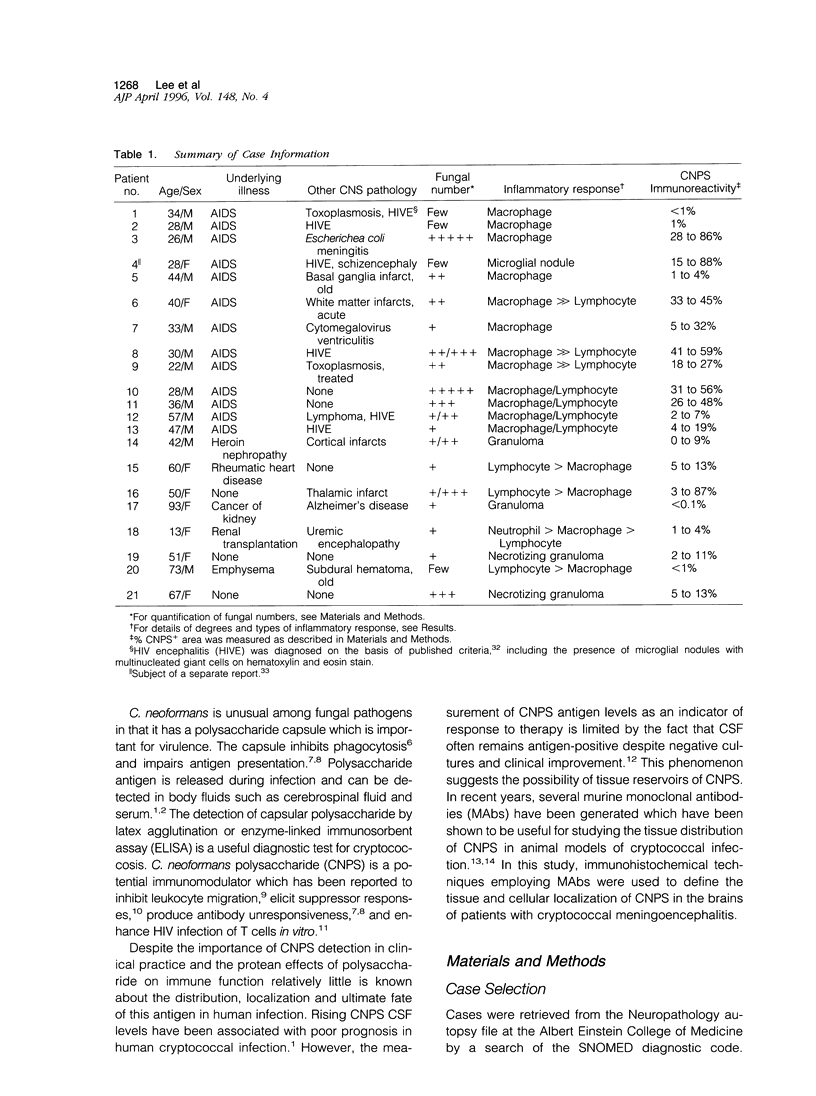
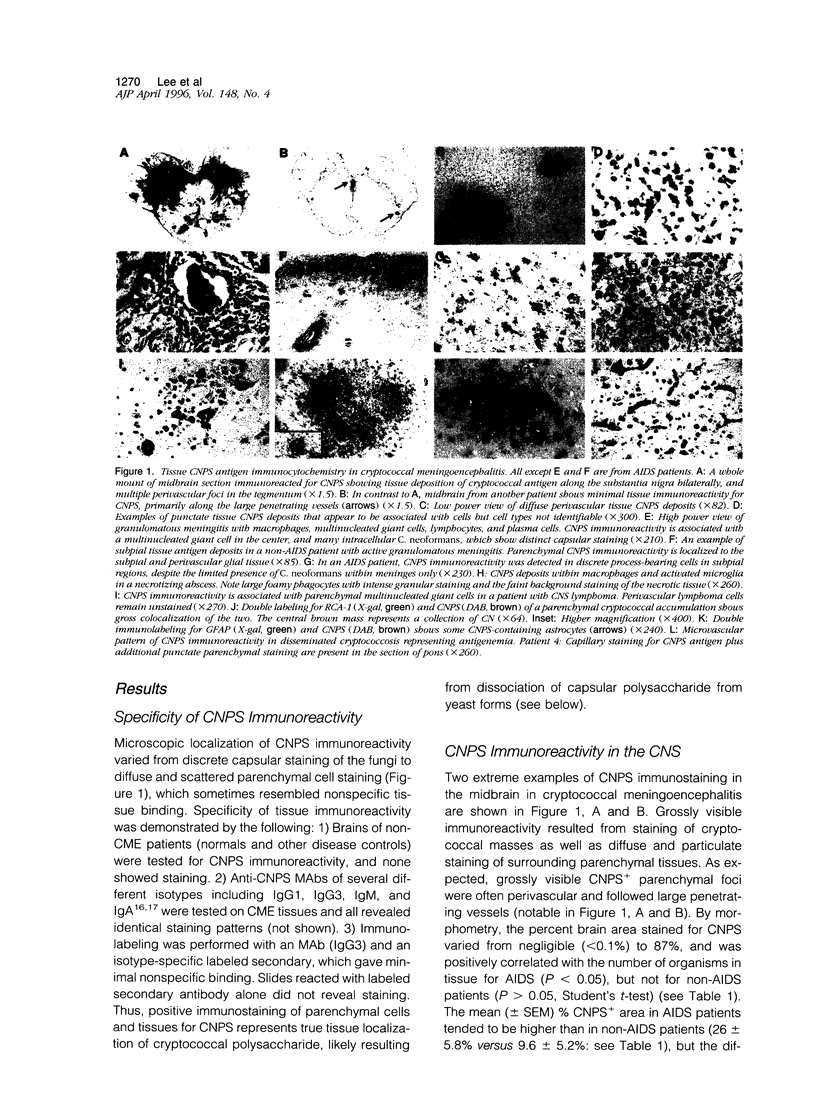
Abstract
Cryptococcal meningoencephalitis (CME) is caused by the encapsulated fungus Cryptococcus neoformans (CN) and is a major cause of mortality and morbidity in patients with AIDS. The polysaccharide capsule of CN is important for virulence, and soluble polysaccharide has the potential to cause immune modulation. To better understand the interactions of central nervous system cells and cryptococcal capsular polysaccharide (CNPS) in the pathogenesis of human CME, postmortem brain tissue from 21 patients with CME (13 AIDS and 8 non-AIDS patients) was analyzed. Histopathology and distribution of tissue CNPS antigen were analyzed using monoclonal antibodies against CNPS in combination with cell type-specific markers (glial fibrillary acidic protein for astrocytes, Ricinus communis agglutinin (RCA)-l for macrophage/microglia and endothelial cells; UCHL-1 for T cells, L26 for B cells). The CN cells showed discrete capsular immunoreactivity as expected; however, diffuse and particulate cellular and tissue staining for CNPS was detected in the brain parenchyma and the meninges in all cases. By quantitative analysis, the CNPS immunoreactive area ranged from 0.1 to 88 percent of tissue cross sectional area, and tended to be higher in brains of AIDS (median values from two sections ranged from 1 to 57 percent; mean, 26 percent) than in non-AIDS (0.1 to 40 percent; mean, 9.6 percent) patients. The proportion of CNPS immunoreactive area was positively correlated with the estimated number of CN. None (0/13) of the AIDS patients displayed significant inflammatory responses to CN, whereas most (7/8) non-AIDS patients showed granulomatous inflammatory responses. The phenotype of infiltrating lymphocytes was UCHL-1+/L26-/RC4-, thus consistent with activated T cells, both in AIDS and non-AIDS patients. Double immunolabeling studies revealed that tissue CNPS immunoreactivity was most often localized in macrophages and microglia, less frequently in reactive astrocytes and endothelial cells, but not in lymphocytes. This study demonstrates that CNPS can be detected not only in the serum and cerebrospinal fluid (CSF) of patients, but also in the affected tissue, most often localized in cells of mononuclear phagocyte system. Potential implications of these findings for the pathogenesis of CME are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders K. H., Guerra W. F., Tomiyasu U., Verity M. A., Vinters H. V. The neuropathology of AIDS. UCLA experience and review. Am J Pathol. 1986 Sep;124(3):537–558. [PMC free article] [PubMed] [Google Scholar]
- BENNETT J. E., HASENCLEVER H. F. CRYPTOCOCCUS NEOFORMANS POLYSACCHARIDE: STUDIES OF SEROLOGIC PROPERTIES AND ROLE IN INFECTION. J Immunol. 1965 Jun;94:916–920. [PubMed] [Google Scholar]
- Casadevall A., Scharff M. D. The mouse antibody response to infection with Cryptococcus neoformans: VH and VL usage in polysaccharide binding antibodies. J Exp Med. 1991 Jul 1;174(1):151–160. doi: 10.1084/jem.174.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C. A., Abel C., Patchett J., Keri J., Yao J. Lectin staining of cultured CNS microglia. J Histochem Cytochem. 1992 Apr;40(4):505–512. doi: 10.1177/40.4.1372634. [DOI] [PubMed] [Google Scholar]
- Currie B. P., Casadevall A. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin Infect Dis. 1994 Dec;19(6):1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Erickson N. F., 3rd Chemotaxis of human neutrophils and monocytes induced by Cryptococcus neoformans. Infect Immun. 1982 Oct;38(1):380–382. doi: 10.1128/iai.38.1.380-382.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Lee S. C., Casadevall A. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect Immun. 1994 Nov;62(11):4755–4761. doi: 10.1128/iai.62.11.4755-4761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRANO A., ZIMMERMAN H. M., LEVINE S. FINE STRUCTURE OF CEREBRAL FLUID ACCUMULATION. V. TRANSFER OF FLUID FROM EXTRACELLULAR TO INTRACELLULAR COMPARTMENTS IN ACUTE PHASE OF CRYPTOCOCCAL POLYSACCHARIDE LESIONS. Arch Neurol. 1964 Dec;11:632–641. doi: 10.1001/archneur.1964.00460240064009. [DOI] [PubMed] [Google Scholar]
- HIRANO A., ZIMMERMAN H. M., LEVINE S. FINE STRUCTURE OF CEREBRAL FLUID ACCUMULATION. VI. INTRACELLULAR ACCUMULATION OF FLUID AND CRYPTOCOCCAL POLYSACCHARIDE IN OLIGODENDROGLIA. Arch Neurol. 1965 Feb;12:189–196. doi: 10.1001/archneur.1965.00460260079009. [DOI] [PubMed] [Google Scholar]
- Henderson D. K., Bennett J. E., Huber M. A. Long-lasting, specific immunologic unresponsiveness associated with cryptococcal meningitis. J Clin Invest. 1982 May;69(5):1185–1190. doi: 10.1172/JCI110555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- Kozel T. R., Gulley W. F., Cazin J., Jr Immune response to Cryptococcus neoformans soluble polysaccharide: immunological unresponsiveness. Infect Immun. 1977 Dec;18(3):701–707. doi: 10.1128/iai.18.3.701-707.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure K., Lyman W. D., Weidenheim K. M., Dickson D. W. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am J Pathol. 1990 May;136(5):1085–1092. [PMC free article] [PubMed] [Google Scholar]
- Kure K., Weidenheim K. M., Lyman W. D., Dickson D. W. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 1990;80(4):393–400. doi: 10.1007/BF00307693. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Popkin T. J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982 Jun;150(3):1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Rhodes J. C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986 Jan;51(1):218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Shafit-Zagardo B., Aquino D. A., Zhao M. L., Dickson D. W., Brosnan C. F., Lee S. C. Cytoskeletal alterations in human fetal astrocytes induced by interleukin-1 beta. J Neurochem. 1994 Nov;63(5):1625–1634. doi: 10.1046/j.1471-4159.1994.63051625.x. [DOI] [PubMed] [Google Scholar]
- Maddox D. E., Shibata S., Goldstein I. J. Stimulated macrophages express a new glycoprotein receptor reactive with Griffonia simplicifolia I-B4 isolectin. Proc Natl Acad Sci U S A. 1982 Jan;79(1):166–170. doi: 10.1073/pnas.79.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. P., Puck J. In vitro human lymphocyte responses to Cryptococcus neoformans. Evidence for primary and secondary responses in normals and infected subjects. J Immunol. 1984 Jul;133(1):166–172. [PubMed] [Google Scholar]
- Muchmore H. G., Scott E. N., Felton F. G., Fromtling R. A. Cryptococcal capsular polysaccharide clearance in nonimmune mice. Mycopathologia. 1982 Apr 23;78(1):41–45. doi: 10.1007/BF00436580. [DOI] [PubMed] [Google Scholar]
- Mukherjee J., Casadevall A., Scharff M. D. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J Exp Med. 1993 Apr 1;177(4):1105–1116. doi: 10.1084/jem.177.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Lee S., Mukherjee J., Scharff M. D., Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994 Mar;62(3):1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettoello-Mantovani M., Casadevall A., Kollmann T. R., Rubinstein A., Goldstein H. Enhancement of HIV-1 infection by the capsular polysaccharide of Cryptococcus neoformans. Lancet. 1992 Jan 4;339(8784):21–23. doi: 10.1016/0140-6736(92)90142-p. [DOI] [PubMed] [Google Scholar]
- Powderly W. G., Cloud G. A., Dismukes W. E., Saag M. S. Measurement of cryptococcal antigen in serum and cerebrospinal fluid: value in the management of AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1994 May;18(5):789–792. doi: 10.1093/clinids/18.5.789. [DOI] [PubMed] [Google Scholar]
- Wang Y., Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun. 1994 Jul;62(7):3004–3007. doi: 10.1128/iai.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenheim K. M., Epshteyn I., Lyman W. D. Immunocytochemical identification of T-cells in HIV-1 encephalitis: implications for pathogenesis of CNS disease. Mod Pathol. 1993 Mar;6(2):167–174. [PubMed] [Google Scholar]
- Zuger A., Louie E., Holzman R. S., Simberkoff M. S., Rahal J. J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med. 1986 Feb;104(2):234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]



