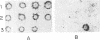Abstract
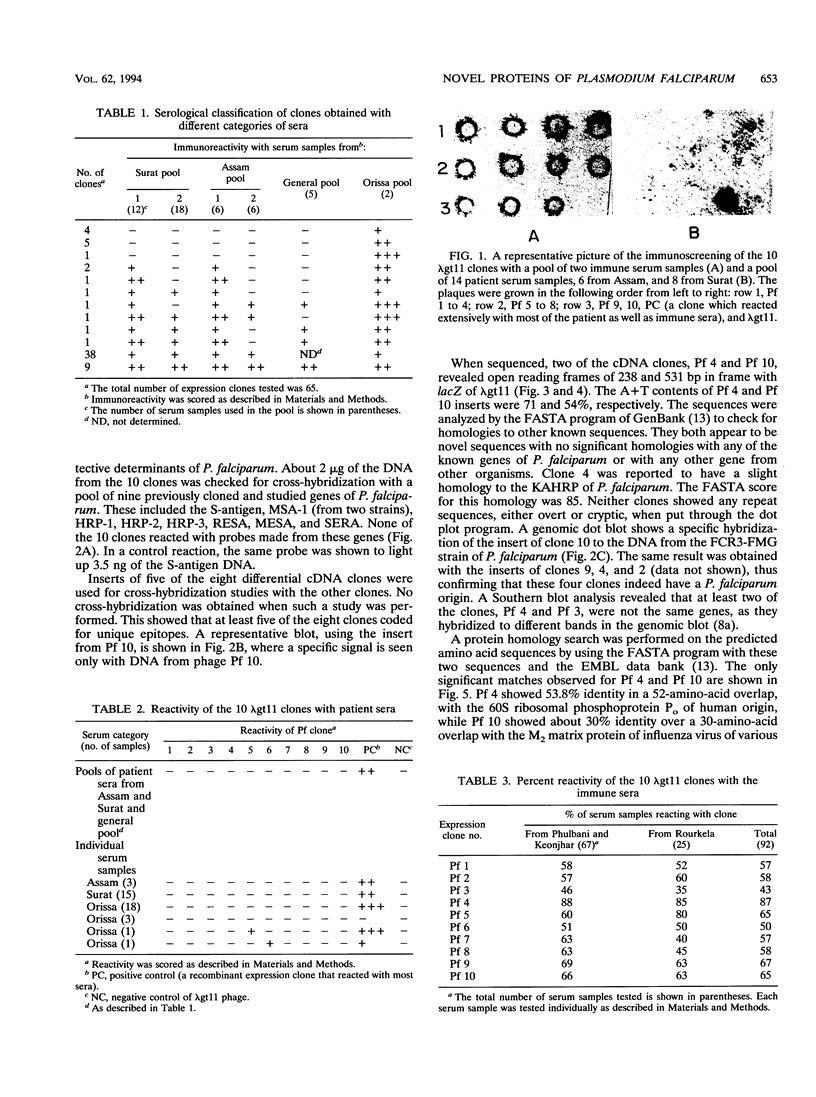
A differential serological screen of a lambda gt11 cDNA expression library of Plasmodium falciparum was performed in an attempt to identify novel and putative host-protective antigens of the parasite. The screening was done with two categories of sera: (i) acute-phase sera obtained from smear-positive acutely infected P. falciparum patients from various regions in India and (ii) immune sera taken from healthy, permanent adult residents of P. falciparum-endemic rural districts of Orissa in eastern India. These adults had not suffered from any clinical malarial symptoms for at least the previous 3 years at the time of serum collection. Sixty-five clones obtained by screening the lambda gt11 library with two immune serum samples were analyzed extensively with a total of 70 acutely infected patient serum samples. Eight of these clones failed to react with any of the patient sera. Each of these eight clones, when tested individually with 92 serum samples from the immune group, reacted with a minimum of 43% of the samples from this category of sera. Thus, these eight epitopes may encode host-protective elements since they are not recognized by antibodies in the patient sera but react exclusively and extensively with the clinically immune set. Sequence analysis of two of these clones reveals that they are novel Plasmodium genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986 Nov;8(6):529–539. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Buckler-White A. J., Naeve C. W., Murphy B. R. Characterization of a gene coding for M proteins which is involved in host range restriction of an avian influenza A virus in monkeys. J Virol. 1986 Feb;57(2):697–700. doi: 10.1128/jvi.57.2.697-700.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Culvenor J. G., Bianco A. E., Crewther P. E., Stahl H. D., Brown G. V., Anders R. F., Kemp D. J. Variable antigen associated with the surface of erythrocytes infected with mature stages of Plasmodium falciparum. Mol Biochem Parasitol. 1986 Sep;20(3):265–277. doi: 10.1016/0166-6851(86)90107-6. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984 Aug 1;160(2):624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Anders R. F. Repetitive proteins and genes of malaria. Annu Rev Microbiol. 1987;41:181–208. doi: 10.1146/annurev.mi.41.100187.001145. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Zebedee S. L., Richardson C. D. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985 Mar;40(3):627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- MILLER M. J. Observations on the natural history of malaria in the semi-resistant West African. Trans R Soc Trop Med Hyg. 1958 Mar;52(2):152–168. doi: 10.1016/0035-9203(58)90036-1. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L. H., Holsinger L. J., Lamb R. A. Influenza virus M2 protein has ion channel activity. Cell. 1992 May 1;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Rich B. E., Steitz J. A. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987 Nov;7(11):4065–4074. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Godson G. N. Isolation of alpha- and beta-tubulin genes of Plasmodium falciparum using a single oligonucleotide probe. Mol Biochem Parasitol. 1990 Mar;39(2):173–182. doi: 10.1016/0166-6851(90)90056-r. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Sim B. K., Lyon J. A., Wolff R. Merozoite surface protein sequence from the Camp strain of the human malaria parasite Plasmodium falciparum. Nucleic Acids Res. 1988 Feb 11;16(3):1206–1206. doi: 10.1093/nar/16.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems T. E., Howard R. J. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zuckerman A., Spira D., Hamburger J. A procedure for the harvesting of mammalian plasmodia. Bull World Health Organ. 1967;37(3):431–436. [PMC free article] [PubMed] [Google Scholar]