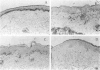
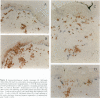
Abstract
Evidence for the involvement of cellular immunity in the etiopathogenesis of the hypopigmentary disorder vitiligo is provided by rare cases of inflammatory vitiligo. Nonlesional, perilesional, and lesional skin biopsies from three inflammatory vitiligo patients were immunohistochemically analyzed. The composition of inflammatory infiltrates present in perilesional skin was analyzed by antibodies to T cells (CD2, CD3, CD4, and CD8), Langerhans cells (CD1a), and macrophages (CD36 and CD68). The presence of activation markers on inflammatory cells was evaluated by analysis of HLA-DR, interleukin-2 receptor, and HECA452 expression. The presence or absence of melanocytes was determined by the antibody NKI-beteb. Moreover, the abundance of matrix molecule tenascin was semi-quantified using T2H5. Results indicate that within perilesional skin, epidermis-infiltrating T cells exhibit an increased CD8/CD4 ratio and increased cutaneous lymphocyte antigen and interleukin-2 receptor expression. These cells are frequently juxtapositionally apposed to remaining melanocytes. In perilesional dermis, CD68+OKM5- macrophages were more numerous than in lesional or nonlesional skin. Keratinocytes as well as melanocytes consistently express major histocompatibility complex class II antigens along stretches of basal and suprabasal layers in perilesional epidermis. Moreover, inflammation is accompanied by increased tenascin content. Although these observations do not permit differentiation between the immune infiltrates being a result as opposed to the cause of the disease process, results presented in this study are very suggestive of involvement of local immune reactivity in melanocyte destruction.
Full text
PDF

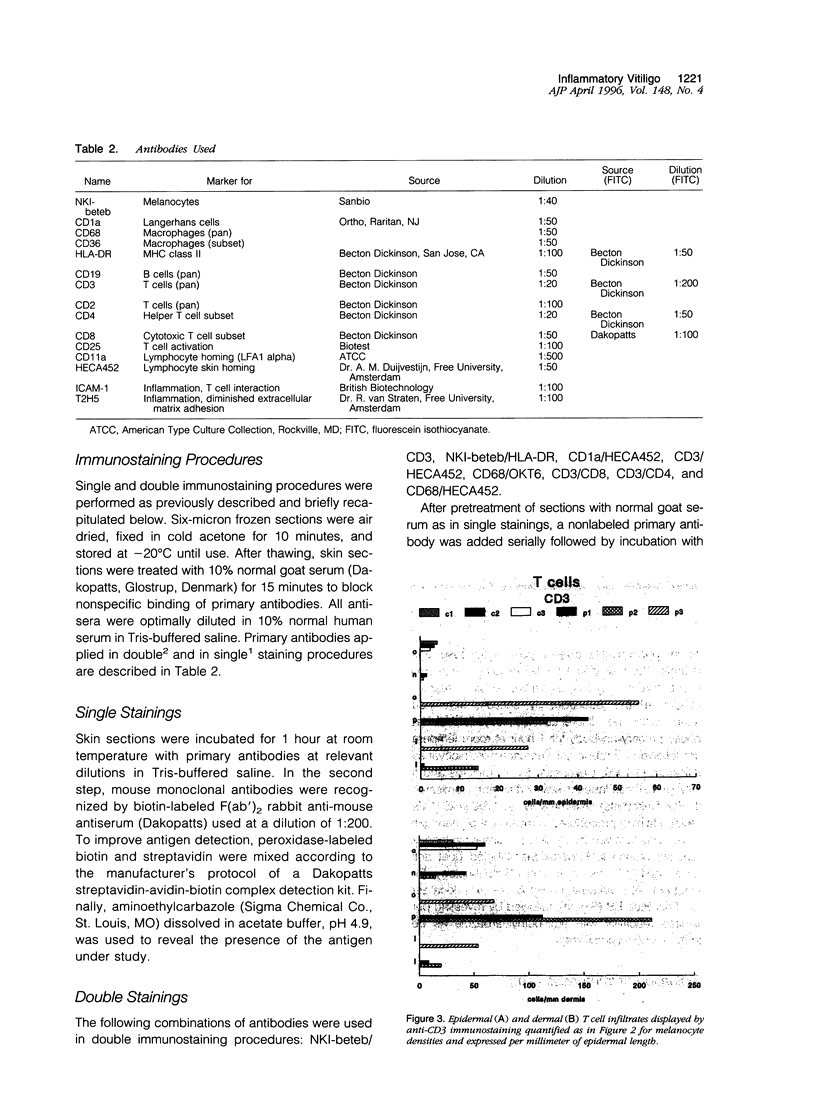




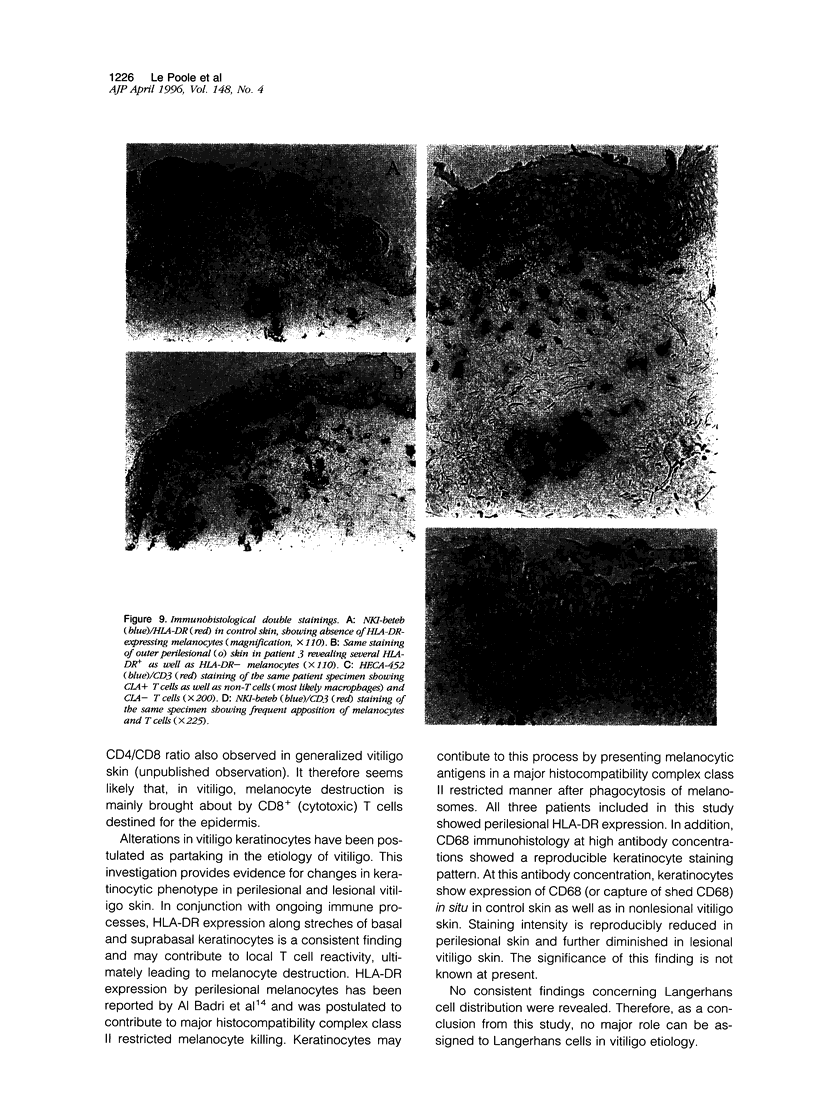


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Naser M. B., Krüger-Krasagakes S., Krasagakis K., Gollnick H., Abdel-Fattah A., Orfanos C. E. Further evidence for involvement of both cell mediated and humoral immunity in generalized vitiligo. Pigment Cell Res. 1994 Feb;7(1):1–8. doi: 10.1111/j.1600-0749.1994.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Abdel-Naser M. B., Ludwig W. D., Gollnick H., Orfanos C. E. Nonsegmental vitiligo: decrease of the CD45RA+ T-cell subset and evidence for peripheral T-cell activation. Int J Dermatol. 1992 May;31(5):321–326. doi: 10.1111/j.1365-4362.1992.tb03946.x. [DOI] [PubMed] [Google Scholar]
- BUCKLEY W. R., LOBITZ W. C., Jr [Vitiligo with a raised inflammatory border]. AMA Arch Derm Syphilol. 1953 Mar;67(3):316–320. doi: 10.1001/archderm.1953.01540030079011. [DOI] [PubMed] [Google Scholar]
- Bakker A. B., Schreurs M. W., de Boer A. J., Kawakami Y., Rosenberg S. A., Adema G. J., Figdor C. G. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994 Mar 1;179(3):1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. D., Hagenaars C., Das P. K., Krieg S. R., Voorn W. J., Kapsenberg M. L. Predominance of "memory" T cells (CD4+, CDw29+) over "naive" T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281(1):24–30. doi: 10.1007/BF00424268. [DOI] [PubMed] [Google Scholar]
- Brichard V., Van Pel A., Wölfel T., Wölfel C., De Plaen E., Lethé B., Coulie P., Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993 Aug 1;178(2):489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galadari E., Mehregan A. H., Hashimoto K. Ultrastructural study of vitiligo. Int J Dermatol. 1993 Apr;32(4):269–271. doi: 10.1111/ijd.1993.32.4.269. [DOI] [PubMed] [Google Scholar]
- Harning R., Cui J., Bystryn J. C. Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol. 1991 Dec;97(6):1078–1080. doi: 10.1111/1523-1747.ep12492607. [DOI] [PubMed] [Google Scholar]
- Insuring against AIDS. Lancet. 1991 Aug 3;338(8762):306–306. [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Rivoltini L., Topalian S. L., Miki T., Rosenberg S. A. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Sakaguchi K., Appella E., Yannelli J. R., Adema G. J., Miki T., Rosenberg S. A. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poole I. C., Das P. K., van den Wijngaard R. M., Bos J. D., Westerhof W. Review of the etiopathomechanism of vitiligo: a convergence theory. Exp Dermatol. 1993 Aug;2(4):145–153. doi: 10.1111/j.1600-0625.1993.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Le Poole I. C., van den Wijngaard R. M., Westerhof W., Dutrieux R. P., Das P. K. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993 Jun;100(6):816–822. doi: 10.1111/1523-1747.ep12476645. [DOI] [PubMed] [Google Scholar]
- Lerner A. B. On the etiology of vitiligo and gray hair. Am J Med. 1971 Aug;51(2):141–147. doi: 10.1016/0002-9343(71)90232-4. [DOI] [PubMed] [Google Scholar]
- Michaëlsson G. Vitiligo with raised borders. Report of two cases. Acta Derm Venereol. 1968;48(2):158–161. [PubMed] [Google Scholar]
- Robbins P. F., el-Gamil M., Kawakami Y., Stevens E., Yannelli J. R., Rosenberg S. A. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994 Jun 15;54(12):3124–3126. [PubMed] [Google Scholar]
- Wölfel T., Hauer M., Klehmann E., Brichard V., Ackermann B., Knuth A., Boon T., Meyer Zum Büschenfelde K. H. Analysis of antigens recognized on human melanoma cells by A2-restricted cytolytic T lymphocytes (CTL). Int J Cancer. 1993 Sep 9;55(2):237–244. doi: 10.1002/ijc.2910550212. [DOI] [PubMed] [Google Scholar]
- al Badri A. M., Foulis A. K., Todd P. M., Gariouch J. J., Gudgeon J. E., Stewart D. G., Gracie J. A., Goudie R. B. Abnormal expression of MHC class II and ICAM-1 by melanocytes in vitiligo. J Pathol. 1993 Feb;169(2):203–206. doi: 10.1002/path.1711690205. [DOI] [PubMed] [Google Scholar]
- de Boer O. J., Verhagen C. E., Visser A., Bos J. D., Das P. K. Cellular interactions and adhesion molecules in psoriatic skin. Acta Derm Venereol Suppl (Stockh) 1994;186:15–18. [PubMed] [Google Scholar]