Abstract
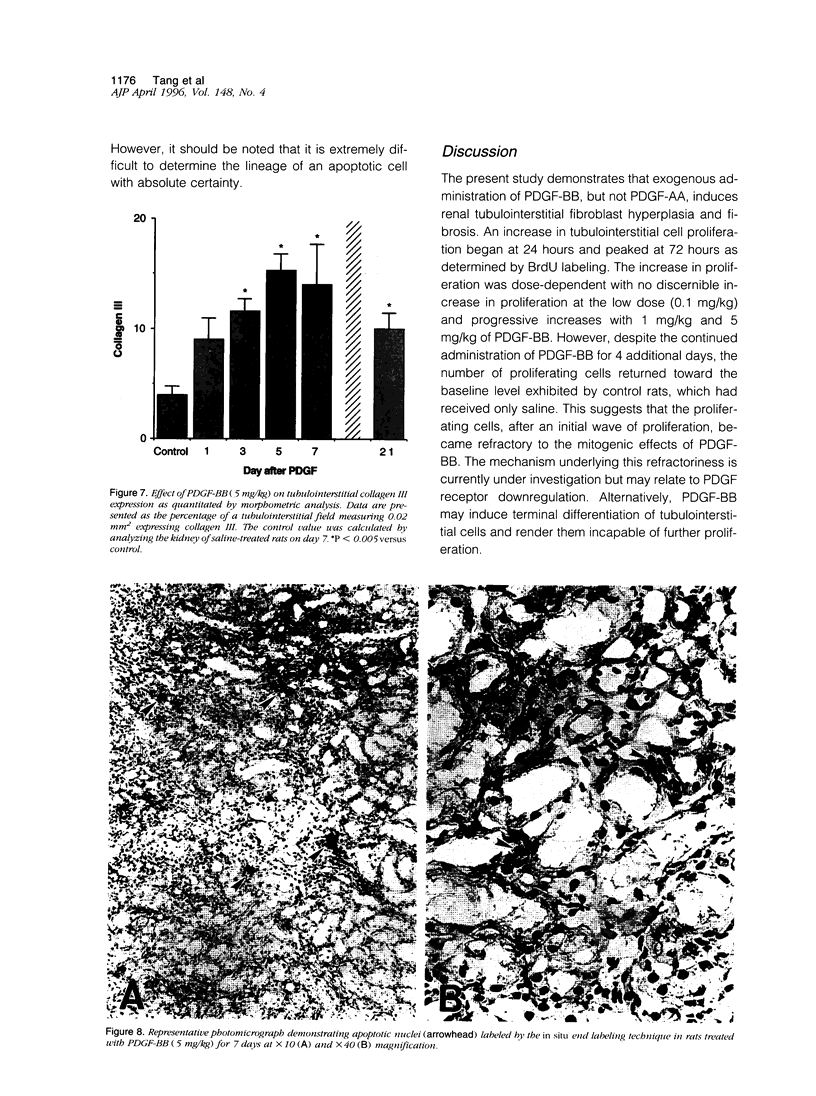
Tubulointerstitial fibrosis correlates closely with renal function, although the mechanism regulating tubulointerstitial fibrogenesis remains poorly understood. Since platelet-derived growth factor (PDGF) is a growth factor for fibroblasts, we examined the effect of daily (for 7 days) PDGF-AA or PDGF-BB administration on renal tubulointerstitial architecture in rats. PDGF-AA administration at a dose of 5 mg/kg did not affect the renal tubulointerstitium. By comparison, PDGF-BB induced dose-dependent renal tubulointerstitial cell proliferation and fibrosis. At 5 mg/kg, PDGF-BB increased BrdU labeling in tubulointerstitial fibroblasts at 24 hours (19-fold), which peaked at 72 hours (23-fold) with bromodeoxyuridine uptake returning to control values by 7 days. Tubulointerstitial proliferation was associated with the differentiation of these cells into myofibroblasts as evidenced by alpha-smooth muscle actin expression beginning on day 3. The expression of alpha-smooth muscle actin peaked on day 5 and had markedly declined by day 21. In addition, apoptotic cells were identified within the tubulointerstitium on day 3 and progressively increased through day 7, suggesting that the disappearance of myofibroblasts may have occurred through apoptosis. These changes were accompanied by increased expression of alpha 1 (III) collagen mRNA and interstitial accumulation of type III collagen within the renal cortex. By morphometric analysis, an approximately twofold increase in collagen III immunolabeling within the interstitial compartment was evident at 24 hours and peaked on days 5 to 7 (approximately fourfold). These data suggest that PDGF-BB may be an important mediator of tubulointerstitial hyperplasia and fibrosis.
Full text
PDF



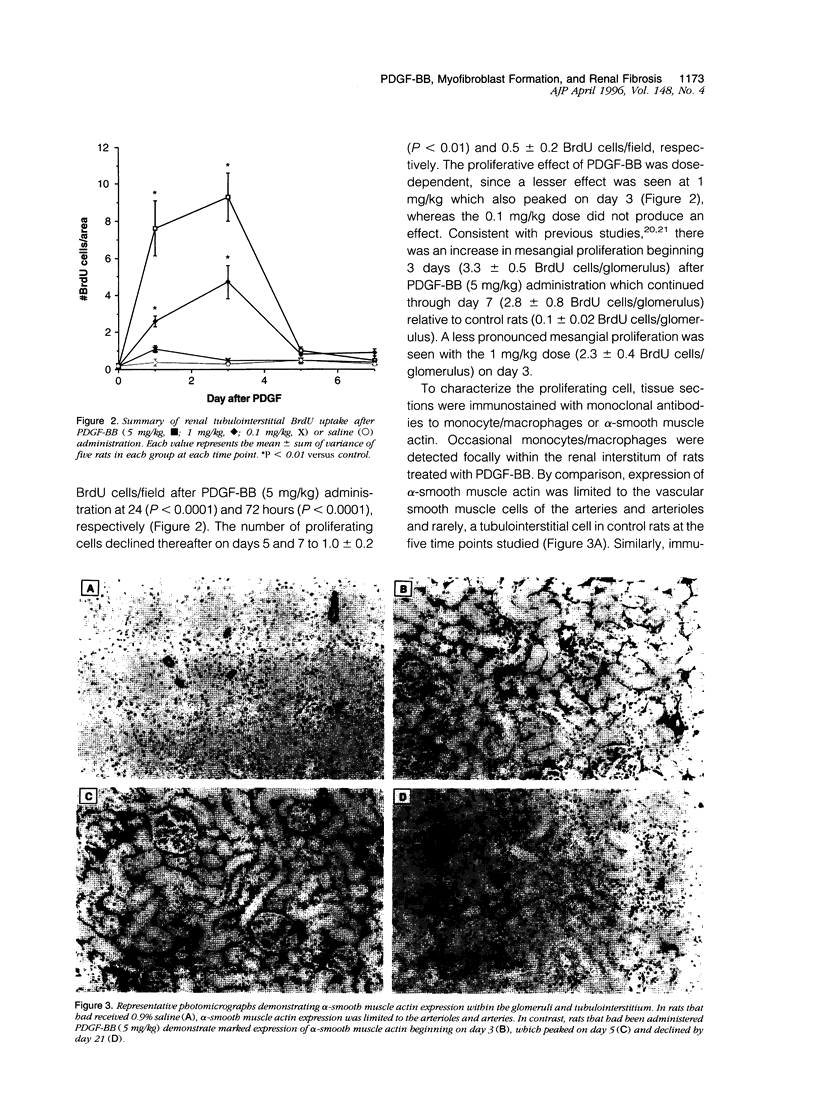
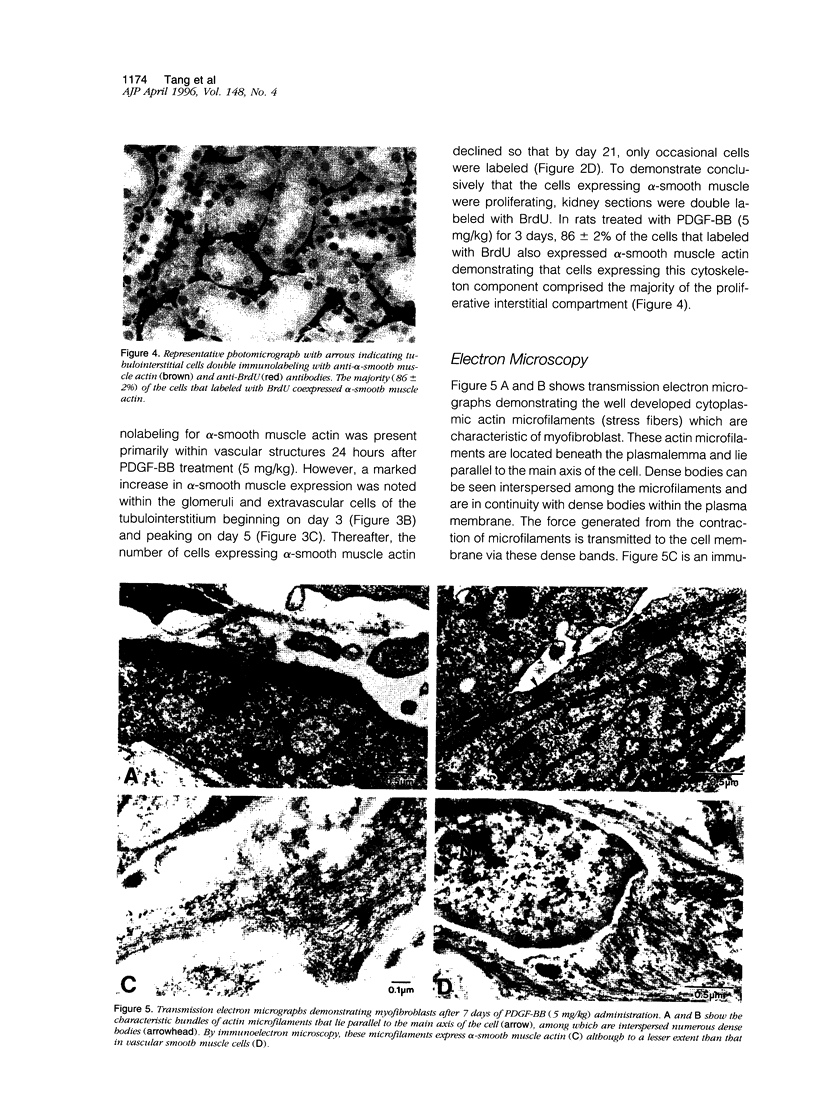
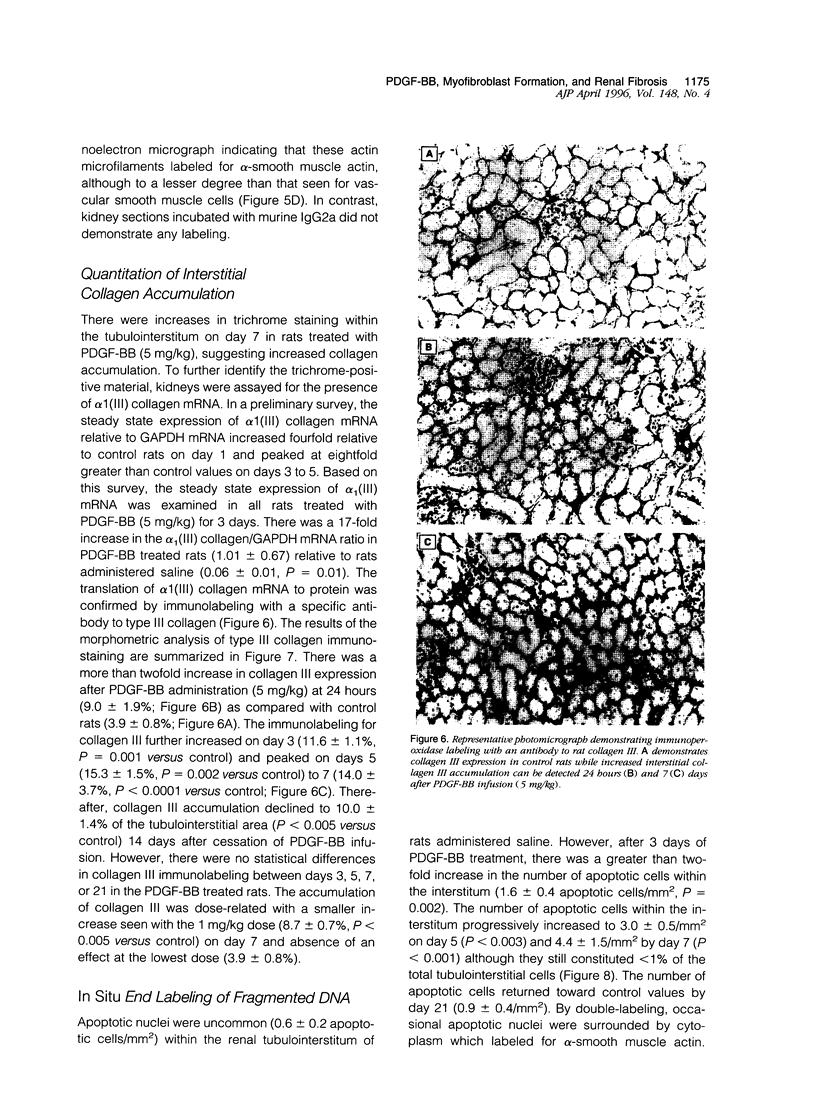




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers C. E., Hudkins K. L., Floege J., Johnson R. J. Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol. 1994 Aug;5(2):201–209. doi: 10.1681/ASN.V52201. [DOI] [PubMed] [Google Scholar]
- Alpers C. E., Seifert R. A., Hudkins K. L., Johnson R. J., Bowen-Pope D. F. PDGF-receptor localizes to mesangial, parietal epithelial, and interstitial cells in human and primate kidneys. Kidney Int. 1993 Feb;43(2):286–294. doi: 10.1038/ki.1993.45. [DOI] [PubMed] [Google Scholar]
- Arora P. D., McCulloch C. A. Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol. 1994 Apr;159(1):161–175. doi: 10.1002/jcp.1041590120. [DOI] [PubMed] [Google Scholar]
- Barja F., Coughlin C., Belin D., Gabbiani G. Actin isoform synthesis and mRNA levels in quiescent and proliferating rat aortic smooth muscle cells in vivo and in vitro. Lab Invest. 1986 Aug;55(2):226–233. [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Bursch W., Paffe S., Putz B., Barthel G., Schulte-Hermann R. Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis. 1990 May;11(5):847–853. doi: 10.1093/carcin/11.5.847. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995 Jan;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Farrell C. L., Bready J. V., Kaufman S. A., Qian Y. X., Burgess T. L. The uptake and distribution of phosphorothioate oligonucleotides into vascular smooth muscle cells in vitro and in rabbit arteries. Antisense Res Dev. 1995 Fall;5(3):175–183. doi: 10.1089/ard.1995.5.175. [DOI] [PubMed] [Google Scholar]
- Floege J., Eng E., Lindner V., Alpers C. E., Young B. A., Reidy M. A., Johnson R. J. Rat glomerular mesangial cells synthesize basic fibroblast growth factor. Release, upregulated synthesis, and mitogenicity in mesangial proliferative glomerulonephritis. J Clin Invest. 1992 Dec;90(6):2362–2369. doi: 10.1172/JCI116126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Eng E., Young B. A., Alpers C. E., Barrett T. B., Bowen-Pope D. F., Johnson R. J. Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest. 1993 Dec;92(6):2952–2962. doi: 10.1172/JCI116918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R., Hsu C. Y., Myers J. C., Lin E., Lyttle C. R., Komm B., Mohn K. Regulation of alpha 2(I), alpha 1(III), and alpha 2(V) collagen mRNAs by estradiol in the immature rat uterus. DNA. 1988 Jun;7(5):347–354. doi: 10.1089/dna.1.1988.7.347. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The biology of the myofibroblast. Kidney Int. 1992 Mar;41(3):530–532. doi: 10.1038/ki.1992.75. [DOI] [PubMed] [Google Scholar]
- Gesualdo L., Di Paolo S., Milani S., Pinzani M., Grappone C., Ranieri E., Pannarale G., Schena F. P. Expression of platelet-derived growth factor receptors in normal and diseased human kidney. An immunohistochemistry and in situ hybridization study. J Clin Invest. 1994 Jul;94(1):50–58. doi: 10.1172/JCI117348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. R., Chang T., Seppä H. E., Kleinman H. K., Martin G. R. Platelet-derived growth factor is a chemoattractant for vascular smooth muscle cells. J Cell Physiol. 1982 Nov;113(2):261–266. doi: 10.1002/jcp.1041130213. [DOI] [PubMed] [Google Scholar]
- Humes H. D., Beals T. F., Cieslinski D. A., Sanchez I. O., Page T. P. Effects of transforming growth factor-beta, transforming growth factor-alpha, and other growth factors on renal proximal tubule cells. Lab Invest. 1991 Apr;64(4):538–545. [PubMed] [Google Scholar]
- Högemann B., Gillessen A., Böcker W., Rauterberg J., Domschke W. Myofibroblast-like cells produce mRNA for type I and III procollagens in chronic active hepatitis. Scand J Gastroenterol. 1993 Jul;28(7):591–594. doi: 10.3109/00365529309096093. [DOI] [PubMed] [Google Scholar]
- Isaka Y., Fujiwara Y., Ueda N., Kaneda Y., Kamada T., Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993 Dec;92(6):2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Alpers C. E., Yoshimura A., Lombardi D., Pritzl P., Floege J., Schwartz S. M. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992 May;19(5):464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Raines E. W., Floege J., Yoshimura A., Pritzl P., Alpers C., Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992 May 1;175(5):1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncio G. S., Neilson E. G., Haverty T. Mechanisms of tubulointerstitial fibrosis. Kidney Int. 1991 Mar;39(3):550–556. doi: 10.1038/ki.1991.63. [DOI] [PubMed] [Google Scholar]
- Lemley K. V., Kriz W. Anatomy of the renal interstitium. Kidney Int. 1991 Mar;39(3):370–381. doi: 10.1038/ki.1991.49. [DOI] [PubMed] [Google Scholar]
- MacPherson B. R., Leslie K. O., Lizaso K. V., Schwarz J. E. Contractile cells of the kidney in primary glomerular disorders: an immunohistochemical study using an anti-alpha-smooth muscle actin monoclonal antibody. Hum Pathol. 1993 Jul;24(7):710–716. doi: 10.1016/0046-8177(93)90006-3. [DOI] [PubMed] [Google Scholar]
- Mackensen-Haen S., Eissele R., Bohle A. Contribution on the correlation between morphometric parameters gained from the renal cortex and renal function in IgA nephritis. Lab Invest. 1988 Aug;59(2):239–244. [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Kneiser M. R., Bulger R. E., Benditt E. P. Induction of smooth muscle characteristics in renal interstitial fibroblasts during obstructive nephropathy. Lab Invest. 1973 Oct;29(4):422–427. [PubMed] [Google Scholar]
- Narayanan A. S., Page R. C. Biosynthesis and regulation of type V collagen in diploid human fibroblasts. J Biol Chem. 1983 Oct 10;258(19):11694–11699. [PubMed] [Google Scholar]
- Nath K. A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992 Jul;20(1):1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Risdon R. A., Sloper J. C., De Wardener H. E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968 Aug 17;2(7564):363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. L., Akhtar M., Kramer M. S., Raja R. M., Manchanda R., Lazaro N. Evaluation of clearance studies in lupus nephritis. Clin Nephrol. 1974;2(2):47–51. [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Rubbia-Brandt L., Sappino A. P., Gabbiani G. Locally applied GM-CSF induces the accumulation of alpha-smooth muscle actin containing myofibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(2):73–82. doi: 10.1007/BF02899530. [DOI] [PubMed] [Google Scholar]
- Savage K., Siebert E., Swann D. The effect of platelet-derived growth factor on cell division and glycosaminoglycan synthesis by human skin and scar fibroblasts. J Invest Dermatol. 1987 Jul;89(1):93–99. doi: 10.1111/1523-1747.ep12580438. [DOI] [PubMed] [Google Scholar]
- Schainuck L. I., Striker G. E., Cutler R. E., Benditt E. P. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970 Dec;1(4):631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- Schmitt-Gräff A., Desmoulière A., Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch. 1994;425(1):3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- Schwartzman R. A., Cidlowski J. A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993 Apr;14(2):133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Tanaka Y., Marumo F., Sato C. Phenotypic change in portal fibroblasts in biliary fibrosis. Liver. 1994 Apr;14(2):76–82. doi: 10.1111/j.1600-0676.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Tang W. W., Yin S., Wittwer A. J., Qi M. Chemokine gene expression in anti-glomerular basement membrane antibody glomerulonephritis. Am J Physiol. 1995 Sep;269(3 Pt 2):F323–F330. doi: 10.1152/ajprenal.1995.269.3.F323. [DOI] [PubMed] [Google Scholar]
- Vande Berg J. S., Rudolph R., Woodward M. Comparative growth dynamics and morphology between cultured myofibroblasts from granulating wounds and dermal fibroblasts. Am J Pathol. 1984 Feb;114(2):187–200. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Noble N. A., Miller D. E., Border W. A. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int. 1994 Mar;45(3):916–927. doi: 10.1038/ki.1994.122. [DOI] [PubMed] [Google Scholar]
- Yee J., Kuncio G. S., Neilson E. G. Tubulointerstitial injury following glomerulonephritis. Semin Nephrol. 1991 May;11(3):361–366. [PubMed] [Google Scholar]
- Zhang K., Rekhter M. D., Gordon D., Phan S. H. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994 Jul;145(1):114–125. [PMC free article] [PubMed] [Google Scholar]