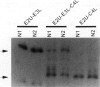
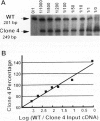
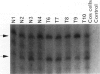
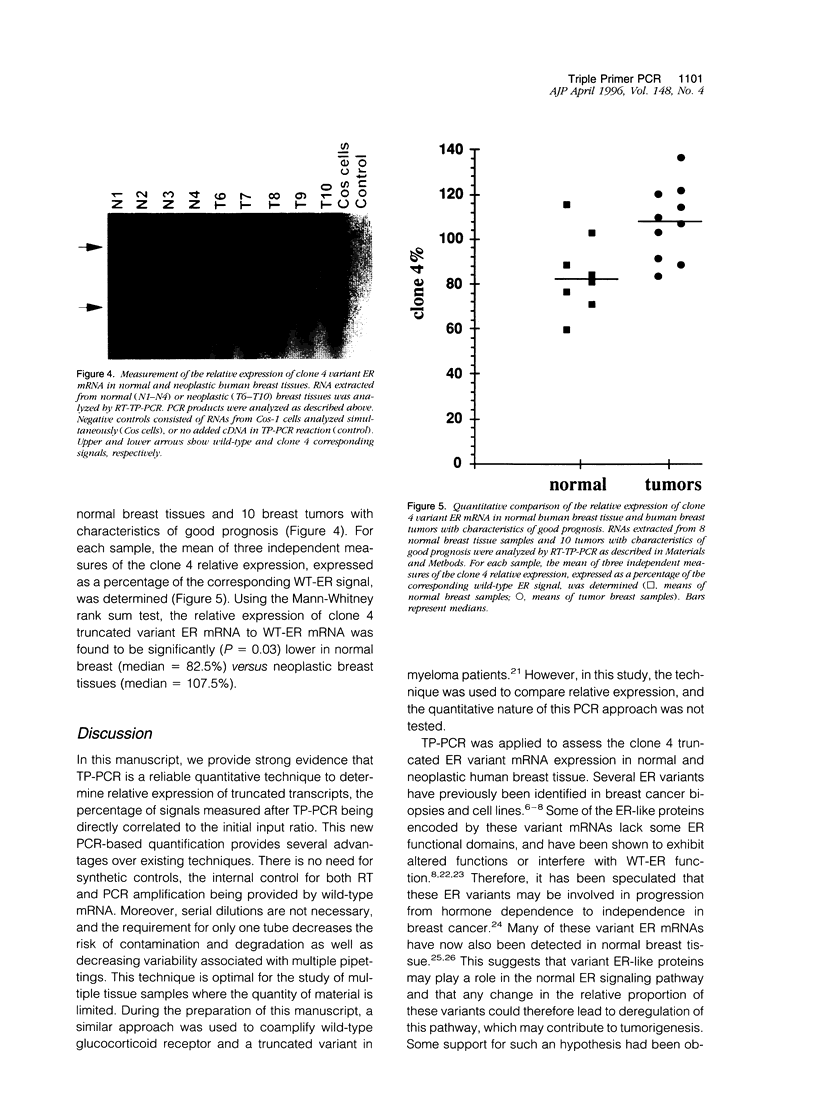
Abstract
The most practical method to quantify mRNA expression within small tumor samples is reverse transcription (RT) followed by quantitative polymerase chain reaction (PCR). One approach, known as "competitive RT-PCR" allows absolute quantitation by reference to synthetic RNA standards but is time-consuming and requires multiple manipulations that limit its usefulness as a screening assay. We describe here a new approach to quantify truncated type mRNAs relative to the wild-type transcripts in small amounts of tissue. This technique, called RT-triple primer-PCR, consists of coamplification of wild-type and truncated cDNAs using three primers in the PCR. To validate this approach, a truncated estrogen receptor variant (clone 4) was quantified relative to the wild-type estrogen receptor using plasmid preparations. The ratio of triple primer-PCR products obtained was directly related to the initial ratio of input cDNAs. RT-triple primer-PCR was then used to compare the relative expression of clone 4 mRNA in frozen sections of normal human breast tissue and human breast tumors with characteristics of good prognosis. The statistically significant difference (P = 0.03) observed between normal and tumor tissues suggests that elevated expression of the clone 4 variant may be associated with early steps of tumorigenesis. This technique provides a useful alternative to already described quantitative RT-PCR techniques for the quantification of truncated mRNA within small amounts of biological material.
Full text
PDF


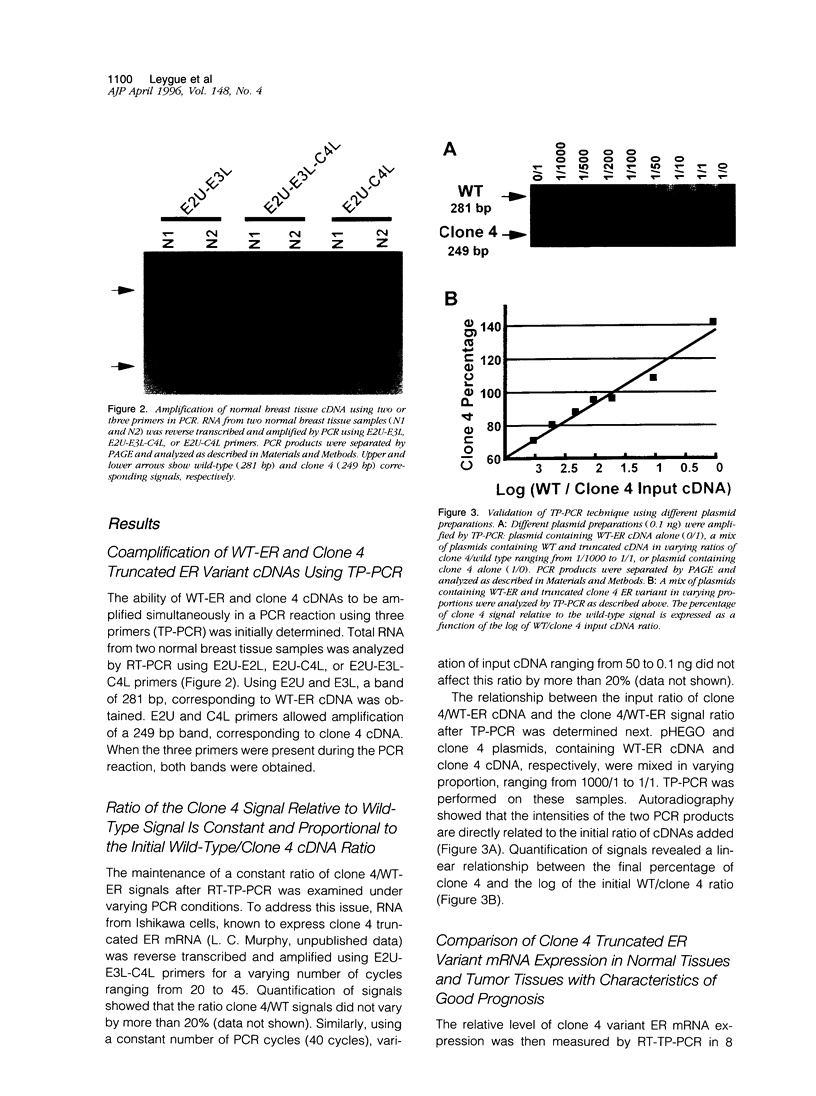


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Chelly J., Gilgenkrantz H., Lambert M., Hamard G., Chafey P., Récan D., Katz P., de la Chapelle A., Koenig M., Ginjaar I. B. Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell. 1990 Dec 21;63(6):1239–1248. doi: 10.1016/0092-8674(90)90419-f. [DOI] [PubMed] [Google Scholar]
- Dotzlaw H., Alkhalaf M., Murphy L. C. Characterization of estrogen receptor variant mRNAs from human breast cancers. Mol Endocrinol. 1992 May;6(5):773–785. doi: 10.1210/mend.6.5.1603086. [DOI] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H., Gläser B., Dreyer C. A naturally occurring short variant of the FTZ-F1-related nuclear orphan receptor xFF1rA and interactions between domains of xFF1rA. Mol Endocrinol. 1995 Jul;9(7):872–886. doi: 10.1210/mend.9.7.7476970. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Allred D. C., Elledge R. M., Krieg S. L., Benedix M. G., Nawaz Z., O'Malley B. W., Greene G. L., McGuire W. L. The ER-positive/PgR-negative breast cancer phenotype is not associated with mutations within the DNA binding domain. Breast Cancer Res Treat. 1993;26(2):191–202. doi: 10.1007/BF00689692. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Fitzgerald S. D., Allred D. C., Elledge R. M., Nawaz Z., McDonnell D. P., O'Malley B. W., Greene G. L., McGuire W. L. Inhibition of estrogen receptor action by a naturally occurring variant in human breast tumors. Cancer Res. 1992 Jan 15;52(2):483–486. [PubMed] [Google Scholar]
- Fuqua S. A., Fitzgerald S. D., Chamness G. C., Tandon A. K., McDonnell D. P., Nawaz Z., O'Malley B. W., McGuire W. L. Variant human breast tumor estrogen receptor with constitutive transcriptional activity. Cancer Res. 1991 Jan 1;51(1):105–109. [PubMed] [Google Scholar]
- Garcia T., Lehrer S., Bloomer W. D., Schachter B. A variant estrogen receptor messenger ribonucleic acid is associated with reduced levels of estrogen binding in human mammary tumors. Mol Endocrinol. 1988 Sep;2(9):785–791. doi: 10.1210/mend-2-9-785. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Ikonen E., Aula P., Grön K., Tollersrud O., Halila R., Manninen T., Syvänen A. C., Peltonen L. Spectrum of mutations in aspartylglucosaminuria. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11222–11226. doi: 10.1073/pnas.88.24.11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Dietz H. C., Nurden A., Bray P. F. Single-strand conformation polymorphism analysis is a rapid and effective method for the identification of mutations and polymorphisms in the gene for glycoprotein IIIa. Blood. 1993 Oct 15;82(8):2281–2288. [PubMed] [Google Scholar]
- Krett N. L., Pillay S., Moalli P. A., Greipp P. R., Rosen S. T. A variant glucocorticoid receptor messenger RNA is expressed in multiple myeloma patients. Cancer Res. 1995 Jul 1;55(13):2727–2729. [PubMed] [Google Scholar]
- Lévesque G., Lamarche B., Murthy M. R., Julien P., Després J. P., Deshaies Y. Determination of changes in specific gene expression by reverse transcription PCR using interspecies mRNAs as internal standards. Biotechniques. 1994 Oct;17(4):738–741. [PubMed] [Google Scholar]
- McGuire W. L., Chamness G. C., Fuqua S. A. Abnormal estrogen receptor in clinical breast cancer. J Steroid Biochem Mol Biol. 1992 Sep;43(1-3):243–247. doi: 10.1016/0960-0760(92)90214-4. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Dotzlaw H. Regulation of transforming growth factor alpha and transforming growth factor beta messenger ribonucleic acid abundance in T-47D, human breast cancer cells. Mol Endocrinol. 1989 Apr;3(4):611–617. doi: 10.1210/mend-3-4-611. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Dotzlaw H. Variant estrogen receptor mRNA species detected in human breast cancer biopsy samples. Mol Endocrinol. 1989 Apr;3(4):687–693. doi: 10.1210/mend-3-4-687. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Hilsenbeck S. G., Dotzlaw H., Fuqua S. A. Relationship of clone 4 estrogen receptor variant messenger RNA expression to some known prognostic variables in human breast cancer. Clin Cancer Res. 1995 Feb;1(2):155–159. [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Patriotis C., Makris A., Bear S. E., Tsichlis P. N. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2251–2255. doi: 10.1073/pnas.90.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer U., Fecarotta E., Vidali G. Coexpression of multiple estrogen receptor variant messenger RNAs in normal and neoplastic breast tissues and in MCF-7 cells. Cancer Res. 1995 May 15;55(10):2158–2165. [PubMed] [Google Scholar]
- Scadden D. T., Wang Z., Groopman J. E. Quantitation of plasma human immunodeficiency virus type 1 RNA by competitive polymerase chain reaction. J Infect Dis. 1992 Jun;165(6):1119–1123. doi: 10.1093/infdis/165.6.1119. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. Competitive PCR. Nature. 1992 Oct 8;359(6395):557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- Spanakis E. Determining the specificity of quantitative variation in gene expression. J Natl Cancer Inst. 1995 Aug 2;87(15):1179–1180. doi: 10.1093/jnci/87.15.1179. [DOI] [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Miksicek R. J. Identification of a dominant negative form of the human estrogen receptor. Mol Endocrinol. 1991 Nov;5(11):1707–1715. doi: 10.1210/mend-5-11-1707. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Seto M., Akao Y., Iida S., Nakazawa S., Oshimura M., Takahashi T., Ueda R. Gene rearrangement and truncated mRNA in cell lines with 11q23 translocation. Oncogene. 1993 Feb;8(2):479–485. [PubMed] [Google Scholar]