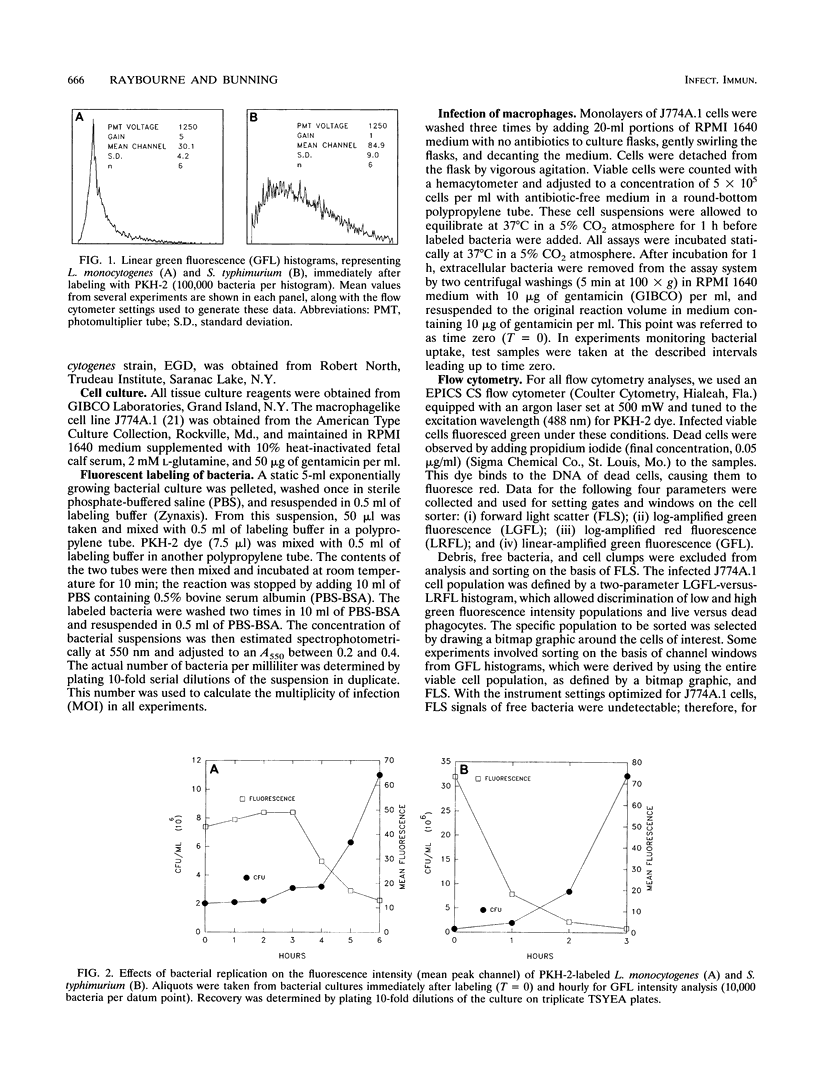
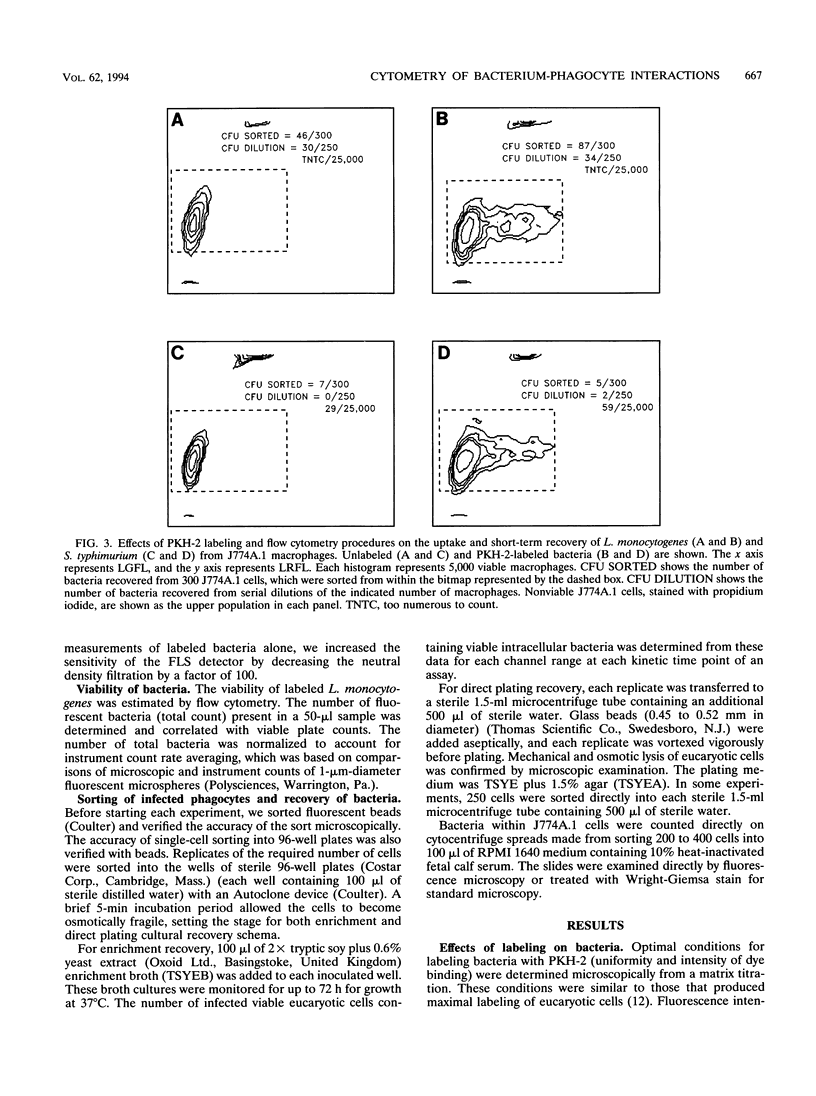
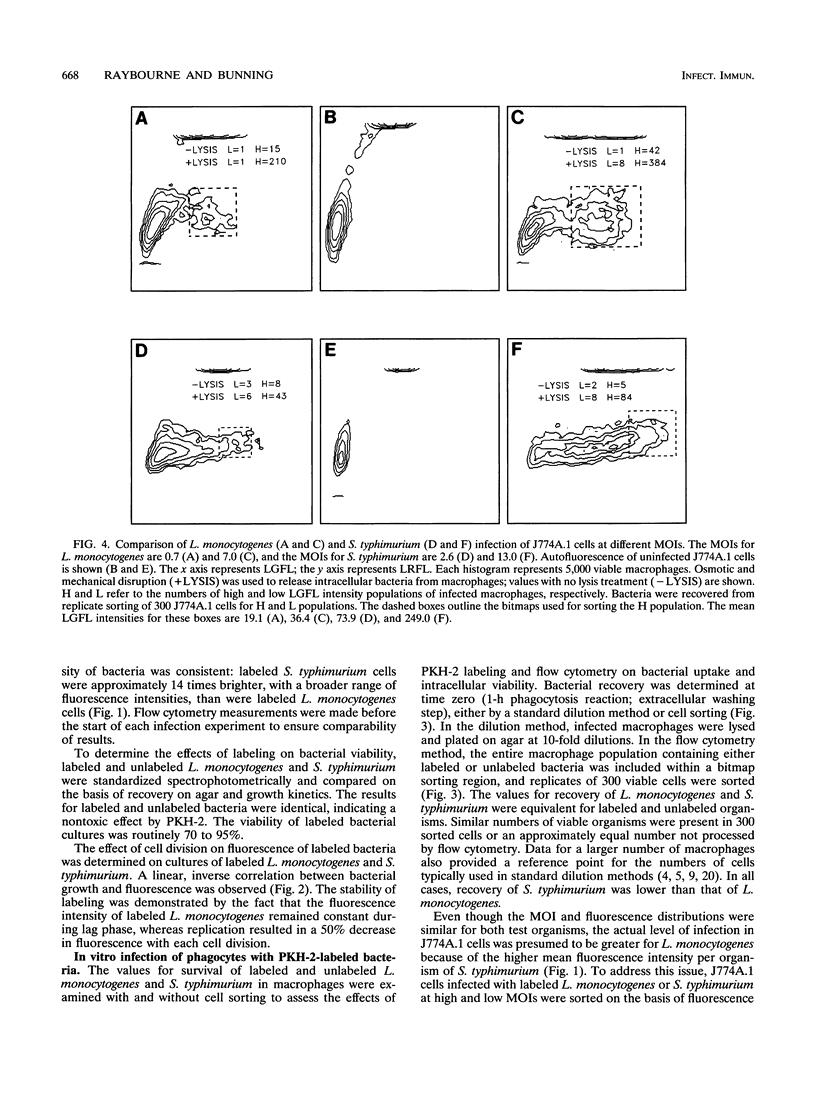
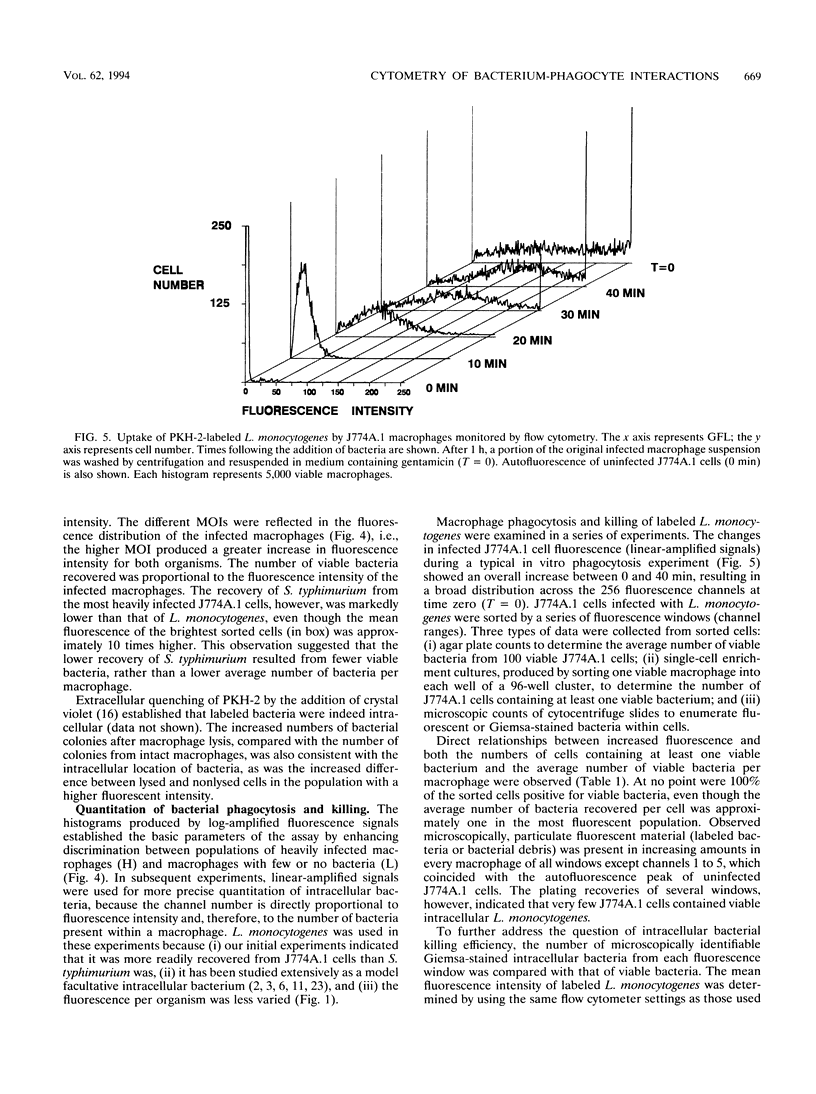
Abstract
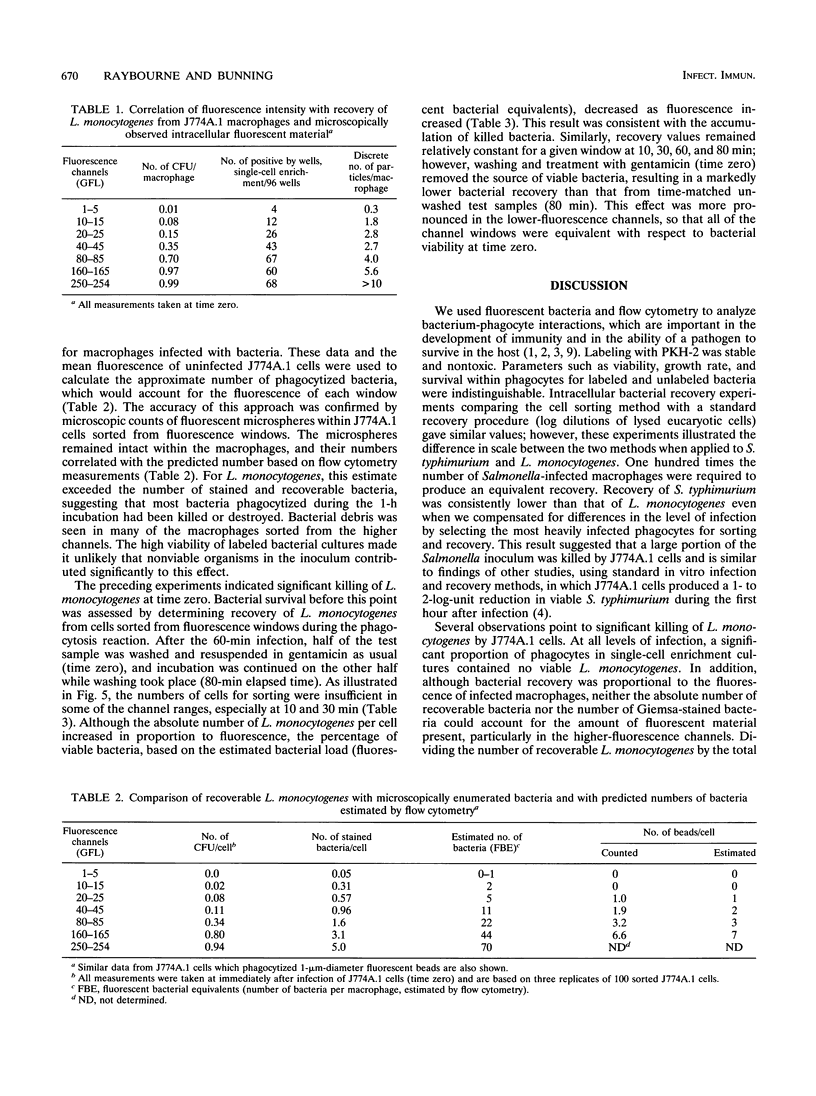
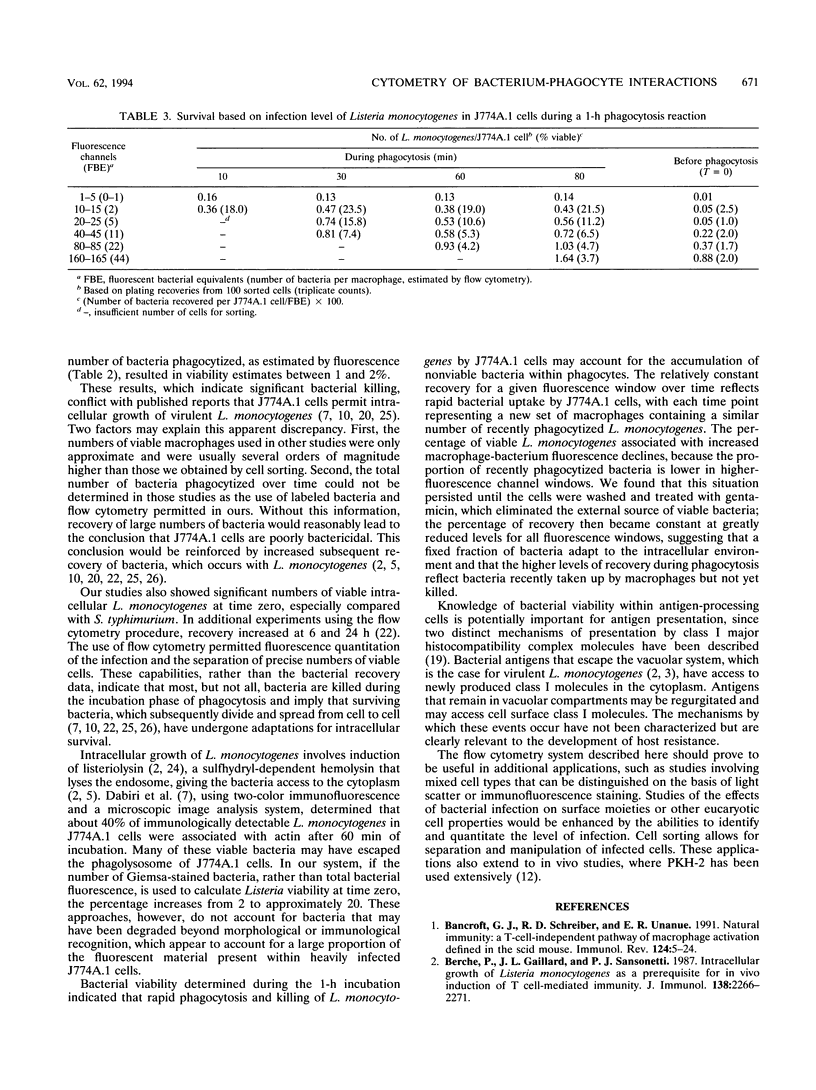
Flow cytometry is a potentially powerful tool for analyzing the interactions of facultative intracellular bacteria and macrophages on a cellular level, particularly when fluorochromes are used to label the bacteria. We labeled Listeria monocytogenes and Salmonella typhimurium with a lipophilic dye, PKH-2, and used flow cytometry to investigate phagocytosis by J774A.1 cells and short-term bacterial survival. Labeled and unlabeled bacteria were identical in terms of viability, growth kinetics, and survival within macrophages, although recovery per macrophage was much greater for L. monocytogenes than for S. typhimurium. Using L. monocytogenes as a prototypical facultative intracellular bacterium, we estimated bacterial survival during phagocytosis on the basis of linear fluorescence measurements of infected J774A.1 cells and recovery of L. monocytogenes from sorted cells. The lower percentage of surviving L. monocytogenes in macrophages containing higher bacterial loads indicated the accumulation of nonviable bacteria within phagocytes. Removal of the external source of viable bacteria by washes and gentamicin treatment reduced the percentage of surviving intracellular L. monocytogenes to a baseline level, and all baseline levels were similar, regardless of bacterial load. Listeria enrichment recoveries, derived from individually sorted J774A.1 cells, demonstrated the heterogeneity of macrophages in intracellular bacterial survival, especially within heavily infected cells. These results indicated that survival of L. monocytogenes was dependent on the adaptations of a small fraction of bacteria within a population of macrophages which permit intracellular growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Schreiber R. D., Unanue E. R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991 Dec;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Sansonetti P. J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987 Apr 1;138(7):2266–2271. [PubMed] [Google Scholar]
- Brunt L. M., Portnoy D. A., Unanue E. R. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990 Dec 1;145(11):3540–3546. [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A., Paynton C. R., Portnoy D. A. Intracellular methicillin selection of Listeria monocytogenes mutants unable to replicate in a macrophage cell line. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5522–5526. doi: 10.1073/pnas.86.14.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Roles of Listeria monocytogenes virulence factors in survival: virulence factors distinct from listeriolysin are needed for the organism to survive an early neutrophil-mediated host defense mechanism. Infect Immun. 1992 Mar;60(3):951–957. doi: 10.1128/iai.60.3.951-957.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri G. A., Sanger J. M., Portnoy D. A., Southwick F. S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C. W., Baigent G. J. Method for flow cytometric detection of Listeria monocytogenes in milk. Appl Environ Microbiol. 1986 Oct;52(4):689–695. doi: 10.1128/aem.52.4.689-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag N. E., Rong L., Portnoy D. A. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect Immun. 1993 Jun;61(6):2537–2544. doi: 10.1128/iai.61.6.2537-2544.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Horan P. K., Melnicoff M. J., Jensen B. D., Slezak S. E. Fluorescent cell labeling for in vivo and in vitro cell tracking. Methods Cell Biol. 1990;33:469–490. doi: 10.1016/s0091-679x(08)60547-6. [DOI] [PubMed] [Google Scholar]
- Horan P. K., Slezak S. E. Stable cell membrane labelling. Nature. 1989 Jul 13;340(6229):167–168. doi: 10.1038/340167a0. [DOI] [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Miliotis M. D. Acridine orange stain for determining intracellular enteropathogens in HeLa cells. J Clin Microbiol. 1991 Apr;29(4):830–831. doi: 10.1128/jcm.29.4.830-831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Mekalanos J. J. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990 May;172(5):2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Safley S. A., Cluff C. W., Marshall N. E., Ziegler H. K. Role of listeriolysin-O (LLO) in the T lymphocyte response to infection with Listeria monocytogenes. Identification of T cell epitopes of LLO. J Immunol. 1991 May 15;146(10):3604–3616. [PubMed] [Google Scholar]
- Sokolovic Z., Fuchs A., Goebel W. Synthesis of species-specific stress proteins by virulent strains of Listeria monocytogenes. Infect Immun. 1990 Nov;58(11):3582–3587. doi: 10.1128/iai.58.11.3582-3587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. N., Camilli A., Portnoy D. A. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1990 Nov;58(11):3770–3778. doi: 10.1128/iai.58.11.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dilla M. A., Langlois R. G., Pinkel D., Yajko D., Hadley W. K. Bacterial characterization by flow cytometry. Science. 1983 May 6;220(4597):620–622. doi: 10.1126/science.6188215. [DOI] [PubMed] [Google Scholar]



