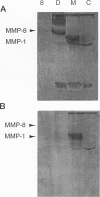
Abstract
A major contribution to the mechanical strength of the heart is provided by a continuous fibrillar collagen network embracing individual myocytes and forming an interstitial and perivascular framework. This study explores the possibility that idiopathic dilated cardiomyopathy may involve extensive changes in this collagenous framework. Idiopathic dilated cardiomyopathy hearts were obtained at transplant and compared with control hearts from autopsy. Idiopathic dilated cardiomyopathy showed a doubling of collagen concentration and a quadrupling of the total collagen per heart, whereas the stable mature cross-link, pyridinoline, diminished from 2.07 mol/mol of collagen to 1.0. Neutrophil-type collagenase activity is elevated approximately 30-fold as is the activity of gelatinase. Tissue inhibitor of metalloproteinase activity falls to negligible levels in idiopathic dilated cardiomyopathy, whereas alpha 2-macroglobulin is high. It is postulated that collagen critical to mechanical stability of the heart is degraded by metalloproteinase activity and is replaced by fibrous intercellular deposits of poorly cross-linked collagen. These changes contribute to weakening and dilatation of the ventricular wall.
Full text
PDF


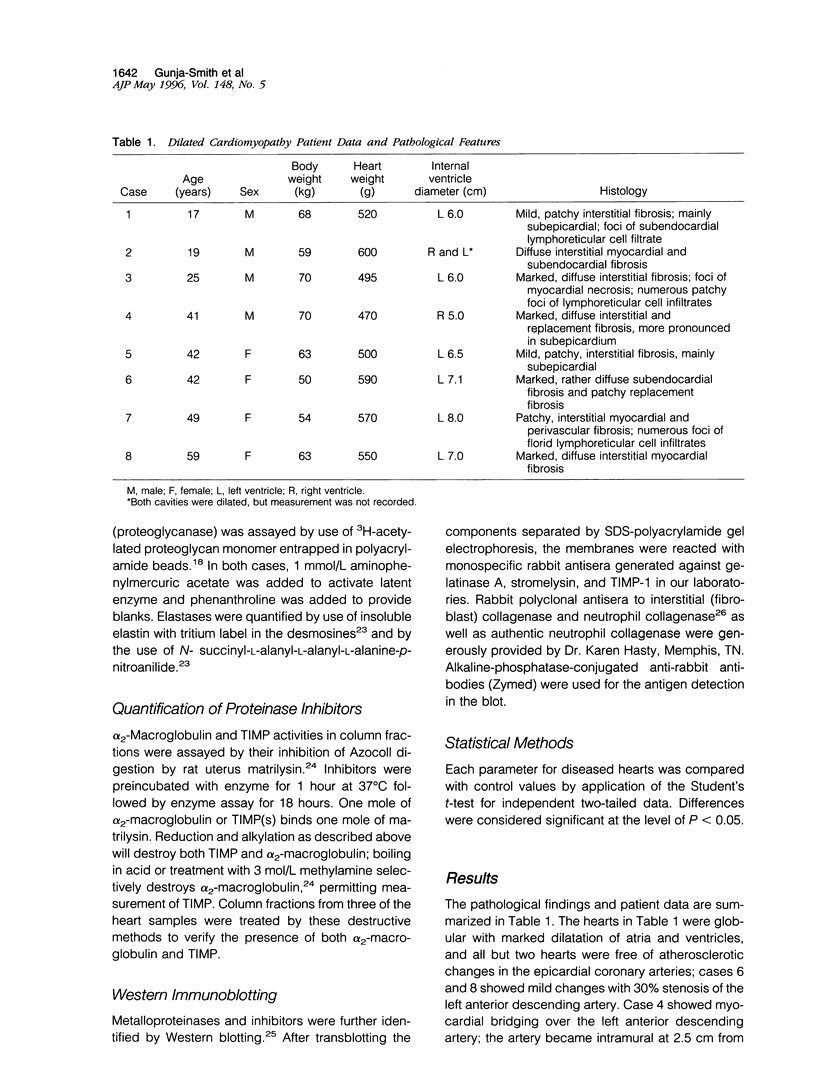

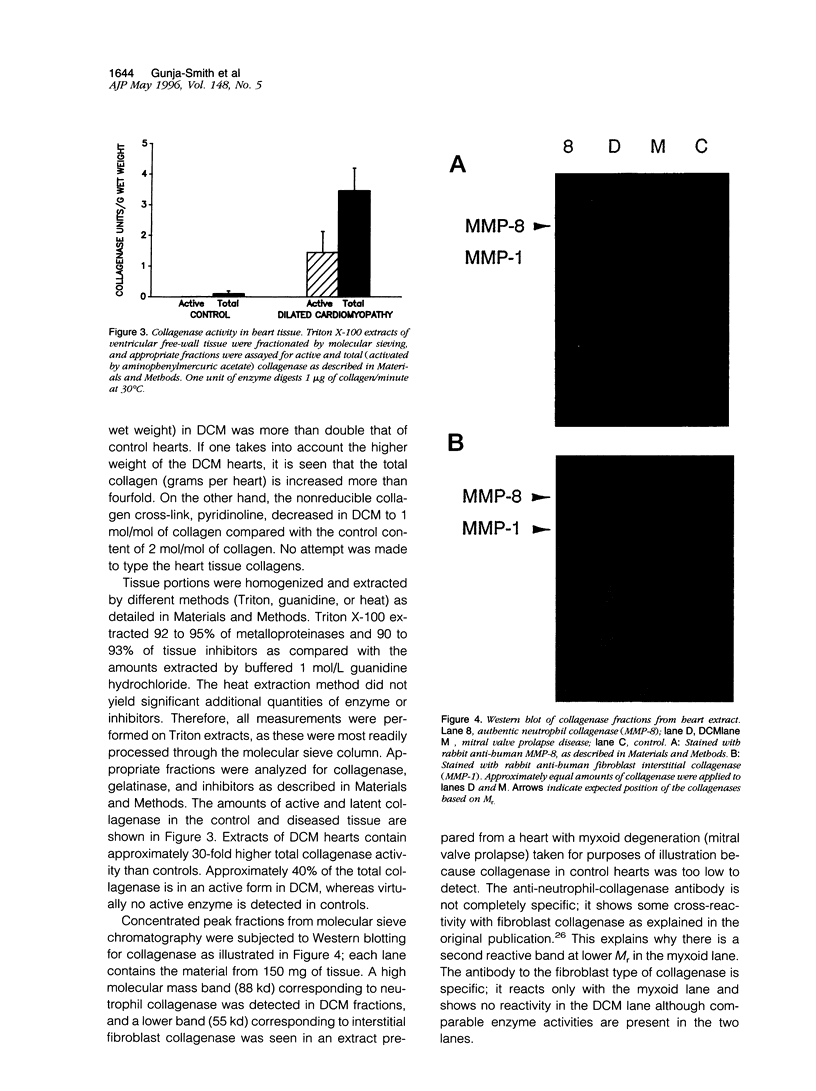
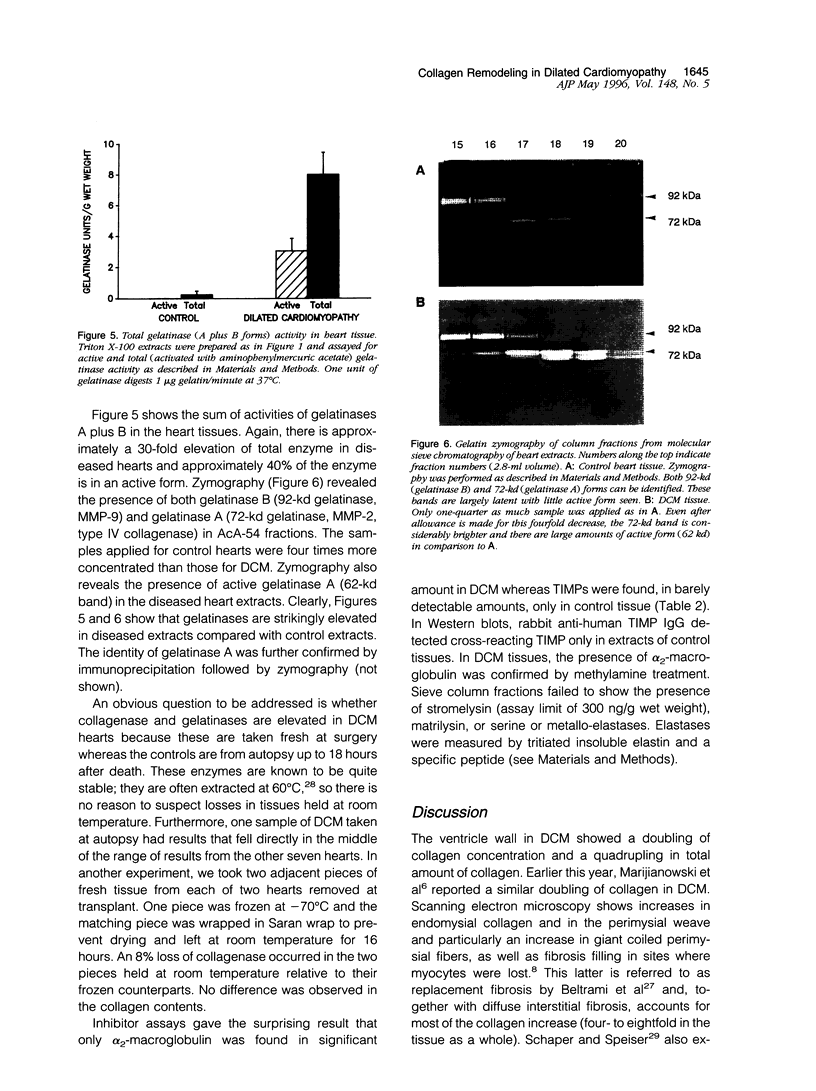



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agren M. S., Taplin C. J., Woessner J. F., Jr, Eaglstein W. H., Mertz P. M. Collagenase in wound healing: effect of wound age and type. J Invest Dermatol. 1992 Dec;99(6):709–714. doi: 10.1111/1523-1747.ep12614202. [DOI] [PubMed] [Google Scholar]
- Beltrami C. A., Finato N., Rocco M., Feruglio G. A., Puricelli C., Cigola E., Sonnenblick E. H., Olivetti G., Anversa P. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995 Jan;27(1):291–305. doi: 10.1016/s0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
- Borg T. K., Caulfield J. B. The collagen matrix of the heart. Fed Proc. 1981 May 15;40(7):2037–2041. [PubMed] [Google Scholar]
- Capasso J. M., Robinson T. F., Anversa P. Alterations in collagen cross-linking impair myocardial contractility in the mouse heart. Circ Res. 1989 Dec;65(6):1657–1664. doi: 10.1161/01.res.65.6.1657. [DOI] [PubMed] [Google Scholar]
- Chakraborty A., Eghbali M. Collagenase activity in the normal rat myocardium. An immunohistochemical method. Histochemistry. 1989;92(5):391–396. doi: 10.1007/BF00492496. [DOI] [PubMed] [Google Scholar]
- Charney R. H., Takahashi S., Zhao M., Sonnenblick E. H., Eng C. Collagen loss in the stunned myocardium. Circulation. 1992 Apr;85(4):1483–1490. doi: 10.1161/01.cir.85.4.1483. [DOI] [PubMed] [Google Scholar]
- Dean D. D., Martel-Pelletier J., Pelletier J. P., Howell D. S., Woessner J. F., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989 Aug;84(2):678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. D., Woessner J. F., Jr A sensitive, specific assay for tissue collagenase using telopeptide-free [3H]acetylated collagen. Anal Biochem. 1985 Jul;148(1):174–181. doi: 10.1016/0003-2697(85)90642-6. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Paz M. A., Gallop P. M. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- Farquharson C., Duncan A., Robins S. P. The effects of copper deficiency on the pyridinium crosslinks of mature collagen in the rat skeleton and cardiovascular system. Proc Soc Exp Biol Med. 1989 Nov;192(2):166–171. doi: 10.3181/00379727-192-42973. [DOI] [PubMed] [Google Scholar]
- Fujimoto D. Isolation and characterization of a fluorescent material in bovine achilles tendon collagen. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1124–1129. doi: 10.1016/0006-291x(77)90972-x. [DOI] [PubMed] [Google Scholar]
- Gabrielescu E., Nicolau N., Butur G., Kozma E., Laky D. Histochemical reactions of myocardial proteases during open heart surgery. Morphol Embryol (Bucur) 1989 Apr-Jun;35(2):103–112. [PubMed] [Google Scholar]
- Gunja-Smith Z. An enzyme-linked immunosorbent assay to quantitate the elastin crosslink desmosine in tissue and urine samples. Anal Biochem. 1985 May 15;147(1):258–264. doi: 10.1016/0003-2697(85)90036-3. [DOI] [PubMed] [Google Scholar]
- Gunja-Smith Z., Lin J., Woessner J. F., Jr Changes in desmosine and pyridinoline crosslinks during rapid synthesis and degradation of elastin and collagen in the rat uterus. Matrix. 1989 Jan;9(1):21–27. doi: 10.1016/s0934-8832(89)80014-9. [DOI] [PubMed] [Google Scholar]
- Gunja-Smith Z., Nagase H., Woessner J. F., Jr Purification of the neutral proteoglycan-degrading metalloproteinase from human articular cartilage tissue and its identification as stromelysin matrix metalloproteinase-3. Biochem J. 1989 Feb 15;258(1):115–119. doi: 10.1042/bj2580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty K. A., Stricklin G. P., Hibbs M. S., Mainardi C. L., Kang A. H. The immunologic relationship of human neutrophil and skin collagenases. Arthritis Rheum. 1987 Jun;30(6):695–699. doi: 10.1002/art.1780300613. [DOI] [PubMed] [Google Scholar]
- Kelly W. A., Kesterson J. W., Carlton W. W. Myocardial lesions in the offspring of female rats fed a copper deficient diet. Exp Mol Pathol. 1974 Feb;20(1):40–56. doi: 10.1016/0014-4800(74)90042-2. [DOI] [PubMed] [Google Scholar]
- Klappacher G., Franzen P., Haab D., Mehrabi M., Binder M., Plesch K., Pacher R., Grimm M., Pribill I., Eichler H. G. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol. 1995 May 1;75(14):913–918. doi: 10.1016/s0002-9149(99)80686-9. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Marijianowski M. M., Teeling P., Mann J., Becker A. E. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol. 1995 May;25(6):1263–1272. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Robinson T. F., Cohen-Gould L., Factor S. M., Eghbali M., Blumenfeld O. O. Structure and function of connective tissue in cardiac muscle: collagen types I and III in endomysial struts and pericellular fibers. Scanning Microsc. 1988 Jun;2(2):1005–1015. [PubMed] [Google Scholar]
- Robinson T. F., Cohen-Gould L., Factor S. M. Skeletal framework of mammalian heart muscle. Arrangement of inter- and pericellular connective tissue structures. Lab Invest. 1983 Oct;49(4):482–498. [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J., Meikle M. C. Neutral metallo-proteinases of rabbit bone. Separation in latent forms of distinct enzymes that when activated degrade collagen, gelatin and proteoglycans. Biochem J. 1978 May 1;171(2):493–496. doi: 10.1042/bj1710493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. J., Franzblau C., Kagan H. M. Proteolysis of insoluble elastin. Methods Enzymol. 1982;82(Pt A):588–605. doi: 10.1016/0076-6879(82)82089-2. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Barry A. C., Factor S. M. Collagen degradation in ischaemic rat hearts. Biochem J. 1990 Jan 1;265(1):233–241. doi: 10.1042/bj2650233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazelaar H. D., Billingham M. E. Leukocytic infiltrates in idiopathic dilated cardiomyopathy. A source of confusion with active myocarditis. Am J Surg Pathol. 1986 Jun;10(6):405–412. doi: 10.1097/00000478-198606000-00005. [DOI] [PubMed] [Google Scholar]
- Tyagi S. C., Matsubara L., Weber K. T. Direct extraction and estimation of collagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin Biochem. 1993 Jun;26(3):191–198. doi: 10.1016/0009-9120(93)90025-2. [DOI] [PubMed] [Google Scholar]
- Weber K. T., Pick R., Janicki J. S., Gadodia G., Lakier J. B. Inadequate collagen tethers in dilated cardiopathy. Am Heart J. 1988 Dec;116(6 Pt 1):1641–1646. doi: 10.1016/0002-8703(88)90763-6. [DOI] [PubMed] [Google Scholar]
- Weber K. T., Sun Y., Tyagi S. C., Cleutjens J. P. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994 Mar;26(3):279–292. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr, Bashey R. I., Boucek R. J. Collagen development in heart and skin of the chick embryo. Biochim Biophys Acta. 1967 Jun 27;140(2):329–338. doi: 10.1016/0005-2795(67)90473-4. [DOI] [PubMed] [Google Scholar]
- Yoshikane H., Honda M., Goto Y., Morioka S., Ooshima A., Moriyama K. Collagen in dilated cardiomyopathy--scanning electron microscopic and immunohistochemical observations. Jpn Circ J. 1992 Sep;56(9):899–910. doi: 10.1253/jcj.56.899. [DOI] [PubMed] [Google Scholar]
- Zhu C., Woessner J. F., Jr A tissue inhibitor of metalloproteinases and alpha-macroglobulins in the ovulating rat ovary: possible regulators of collagen matrix breakdown. Biol Reprod. 1991 Aug;45(2):334–342. doi: 10.1095/biolreprod45.2.334. [DOI] [PubMed] [Google Scholar]