Abstract
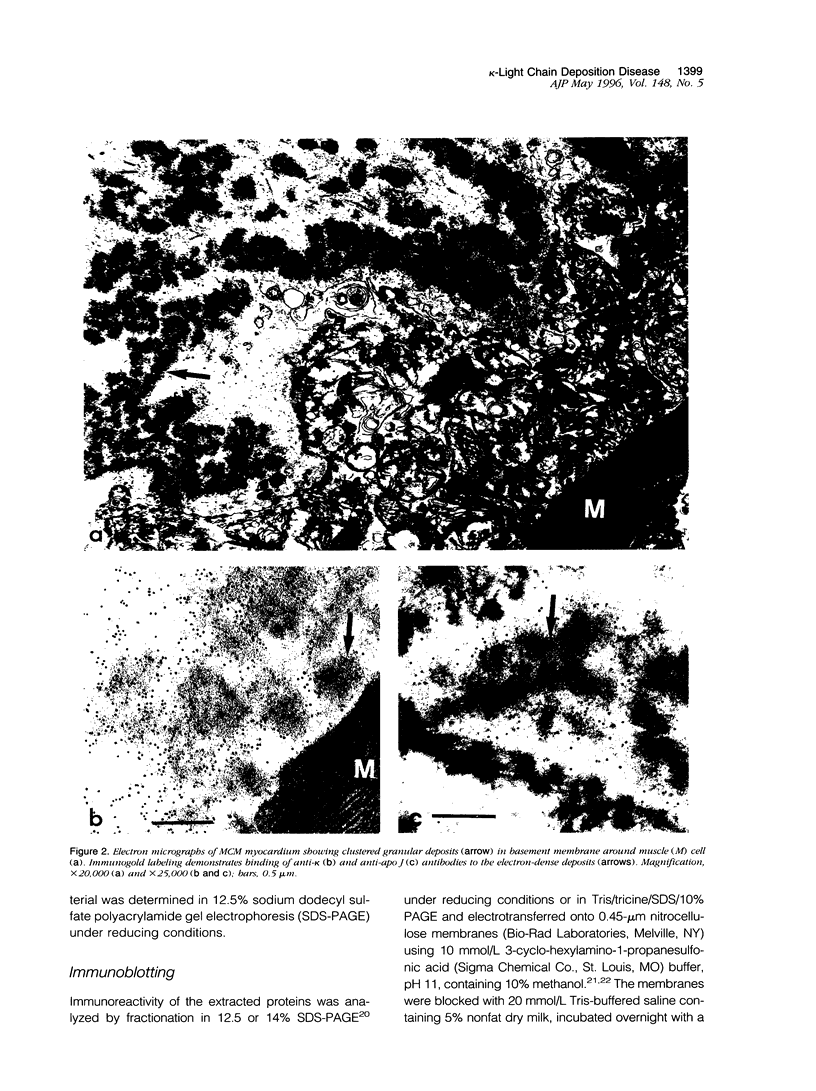
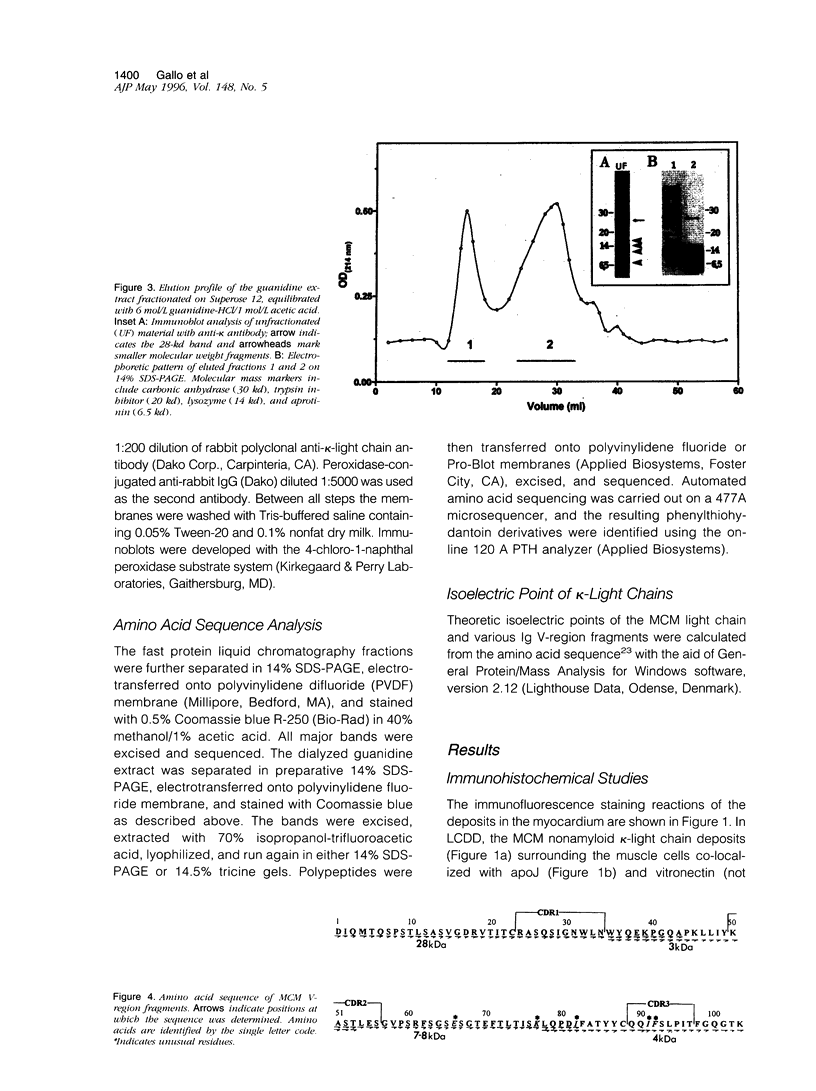
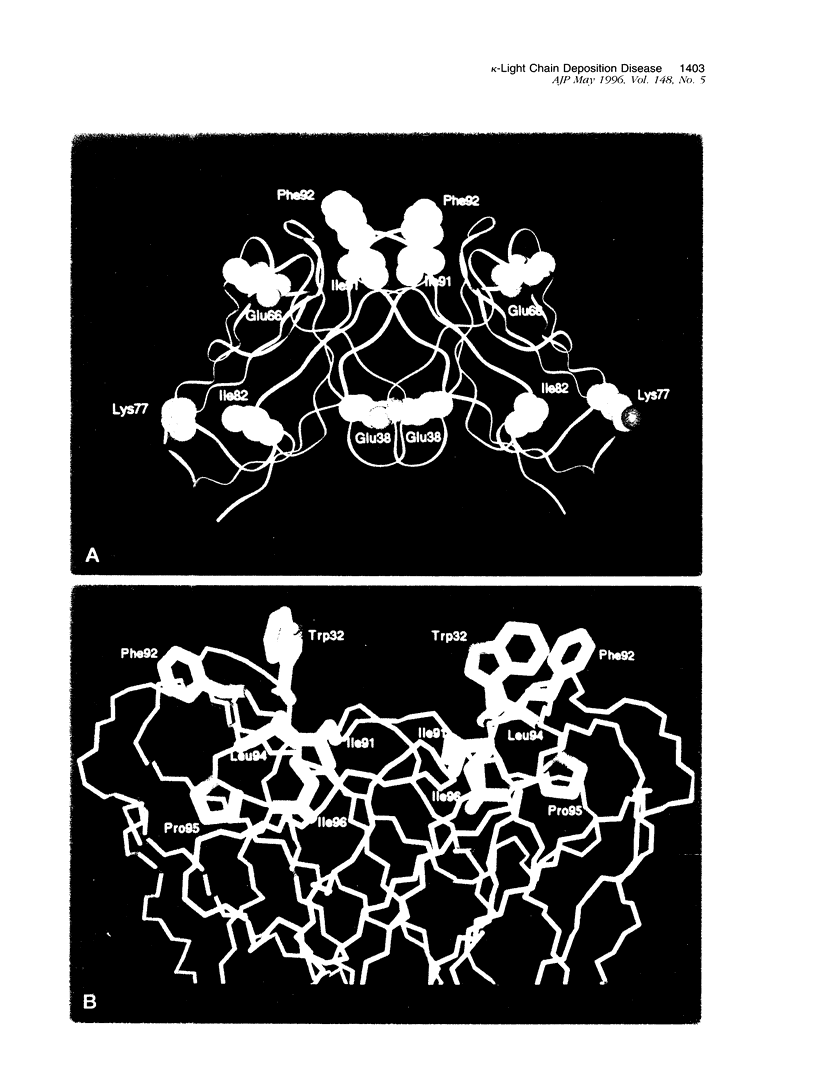
Cardiomyopathy due to monoclonal light chain deposits is a complication of plasma cell disorders. The deposits may be either fibrillar as in light chain amyloid or nonfibrillar as in light chain deposition disease. The reasons for these structural differences are still unknown. We characterized the myocardial deposits by immunohistochemical examination of sections and extraction and biochemical analysis of the tissue deposits in a patient (MCM) who died of myeloma and systemic light chain deposition disease. Amino acid sequence analysis of the extracted nonfibrillar MCM kappa-light chain reveals that it belongs to the L12a germline subset of the kappa(I) protein and contains five distinctive amino acid substitutions (three in the framework region III and two in the complementarity-determining region III) that have not been reported previously in the same positions in other kappa(I) light chains. The theoretically determined isoelectric point (pI 8.21) of the MCM light chain is high compared with the low isoelectric point of other Bence Jones proteins from subjects without light chain deposition disease. The diffuse binding to basement membranes and the high isoelectric point of the MCM kappa-light chain suggest electrostatic interaction as a possible mechanism of tissue deposition. The spatial locations of the five distinctive residues and a sixth rare substitution of the MCM protein modeled on the backbone structure of REI, a kappa(I)-soluble Bence Jones light chain of known three-dimensional structure, may be responsible for protein destabilization, partial unfolding, and aggregation leading to tissue deposition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aucouturier P., Khamlichi A. A., Touchard G., Justrabo E., Cogne M., Chauffert B., Martin F., Preud'homme J. L. Brief report: heavy-chain deposition disease. N Engl J Med. 1993 Nov 4;329(19):1389–1393. doi: 10.1056/NEJM199311043291905. [DOI] [PubMed] [Google Scholar]
- Bellotti V., Merlini G., Bucciarelli E., Perfetti V., Quaglini S., Ascari E. Relevance of class, molecular weight and isoelectric point in predicting human light chain amyloidogenicity. Br J Haematol. 1990 Jan;74(1):65–69. doi: 10.1111/j.1365-2141.1990.tb02539.x. [DOI] [PubMed] [Google Scholar]
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 1-1981. N Engl J Med. 1981 Jan 1;304(1):33–43. doi: 10.1056/NEJM198101013040108. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Cogné M., Preud'homme J. L., Bauwens M., Touchard G., Aucouturier P. Structure of a monoclonal kappa chain of the V kappa IV subgroup in the kidney and plasma cells in light chain deposition disease. J Clin Invest. 1991 Jun;87(6):2186–2190. doi: 10.1172/JCI115252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogné M., Silvain C., Khamlichi A. A., Preud'homme J. L. Structurally abnormal immunoglobulins in human immunoproliferative disorders. Blood. 1992 May 1;79(9):2181–2195. [PubMed] [Google Scholar]
- Dwulet F. E., O'Connor T. P., Benson M. D. Polymorphism in a kappa I primary (AL) amyloid protein (BAN). Mol Immunol. 1986 Jan;23(1):73–78. doi: 10.1016/0161-5890(86)90173-2. [DOI] [PubMed] [Google Scholar]
- Epp O., Lattman E. E., Schiffer M., Huber R., Palm W. The molecular structure of a dimer composed of the variable portions of the Bence-Jones protein REI refined at 2.0-A resolution. Biochemistry. 1975 Nov 4;14(22):4943–4952. doi: 10.1021/bi00693a025. [DOI] [PubMed] [Google Scholar]
- Eulitz M., Linke R. P. Primary structure of the variable part of an amyloidogenic Bence-Jones Protein (Mev.). An unusual insertion in the third hypervariable region of a human kappa-immunoglobulin light chain. Hoppe Seylers Z Physiol Chem. 1982 Nov;363(11):1347–1358. doi: 10.1515/bchm2.1982.363.2.1347. [DOI] [PubMed] [Google Scholar]
- Gallo G. R., Caulin-Glaser T., Lamm M. E. Charge of circulating immune complexes as a factor in glomerular basement membrane localization in mice. J Clin Invest. 1981 May;67(5):1305–1313. doi: 10.1172/JCI110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G., Picken M., Buxbaum J., Frangione B. The spectrum of monoclonal immunoglobulin deposition disease associated with immunocytic dyscrasias. Semin Hematol. 1989 Jul;26(3):234–245. [PubMed] [Google Scholar]
- Gallo G., Wisniewski T., Choi-Miura N. H., Ghiso J., Frangione B. Potential role of apolipoprotein-E in fibrillogenesis. Am J Pathol. 1994 Sep;145(3):526–530. [PMC free article] [PubMed] [Google Scholar]
- Hurle M. R., Helms L. R., Li L., Chan W., Wetzel R. A role for destabilizing amino acid replacements in light-chain amyloidosis. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5446–5450. doi: 10.1073/pnas.91.12.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamlichi A. A., Aucouturier P., Silvain C., Bauwens M., Touchard G., Preud'homme J. L., Nau F., Cogné M. Primary structure of a monoclonal kappa chain in myeloma with light chain deposition disease. Clin Exp Immunol. 1992 Jan;87(1):122–126. doi: 10.1111/j.1365-2249.1992.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Jaenichen R., Zachau H. G. Expressed human immunoglobulin kappa genes and their hypermutation. Eur J Immunol. 1993 Dec;23(12):3248–3262. doi: 10.1002/eji.1830231231. [DOI] [PubMed] [Google Scholar]
- Kyle R. A., Gertz M. A. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995 Jan;32(1):45–59. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makino H., Gibbons J. T., Reddy M. K., Kanwar Y. S. Nephritogenicity of antibodies to proteoglycans of the glomerular basement membrane--I. J Clin Invest. 1986 Jan;77(1):142–156. doi: 10.1172/JCI112269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- May P. C., Finch C. E. Sulfated glycoprotein 2: new relationships of this multifunctional protein to neurodegeneration. Trends Neurosci. 1992 Oct;15(10):391–396. doi: 10.1016/0166-2236(92)90190-j. [DOI] [PubMed] [Google Scholar]
- McAllister H. A., Jr, Seger J., Bossart M., Ferrans V. J. Restrictive cardiomyopathy with kappa light chain deposits in myocardium as a complication of multiple myeloma. Histochemical and electron microscopic observations. Arch Pathol Lab Med. 1988 Nov;112(11):1151–1154. [PubMed] [Google Scholar]
- Murphy B. F., Davies D. J., Morrow W., d'Apice A. J. Localization of terminal complement components S-protein and SP-40,40 in renal biopsies. Pathology. 1989 Oct;21(4):275–278. doi: 10.3109/00313028909061073. [DOI] [PubMed] [Google Scholar]
- Peng S. K., French W. J., Cohen A. H., Fausel R. E. Light chain cardiomyopathy associated with small-vessel disease. Arch Pathol Lab Med. 1988 Aug;112(8):844–846. [PubMed] [Google Scholar]
- Picken M. M., Frangione B., Barlogie B., Luna M., Gallo G. Light chain deposition disease derived from the kappa I light chain subgroup. Biochemical characterization. Am J Pathol. 1989 Apr;134(4):749–754. [PMC free article] [PubMed] [Google Scholar]
- Randall R. E., Williamson W. C., Jr, Mullinax F., Tung M. Y., Still W. J. Manifestations of systemic light chain deposition. Am J Med. 1976 Feb;60(2):293–299. doi: 10.1016/0002-9343(76)90440-x. [DOI] [PubMed] [Google Scholar]
- Rocca A., Khamlichi A. A., Aucouturier P., Noël L. H., Denoroy L., Preud'homme J. L., Cogné M. Primary structure of a variable region of the V kappa I subgroup (ISE) in light chain deposition disease. Clin Exp Immunol. 1993 Mar;91(3):506–509. doi: 10.1111/j.1365-2249.1993.tb05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Wu T. T., Kabat E. A. Subgroups of variable region genes of beta chains of T-cell receptors for antigen. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4461–4463. doi: 10.1073/pnas.83.12.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A., Weiss D. T., Kattine A. A. Nephrotoxic potential of Bence Jones proteins. N Engl J Med. 1991 Jun 27;324(26):1845–1851. doi: 10.1056/NEJM199106273242603. [DOI] [PubMed] [Google Scholar]
- Soto C., Frangione B. Two conformational states of amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neurosci Lett. 1995 Feb 17;186(2-3):115–118. doi: 10.1016/0304-3940(95)11299-c. [DOI] [PubMed] [Google Scholar]
- Staros E., Katz S. M. Myocardial necrosis in light chain deposition. Am Heart J. 1985 Dec;110(6):1295–1296. doi: 10.1016/0002-8703(85)90028-6. [DOI] [PubMed] [Google Scholar]
- Stevens F. J., Myatt E. A., Chang C. H., Westholm F. A., Eulitz M., Weiss D. T., Murphy C., Solomon A., Schiffer M. A molecular model for self-assembly of amyloid fibrils: immunoglobulin light chains. Biochemistry. 1995 Aug 29;34(34):10697–10702. doi: 10.1021/bi00034a001. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda M., Kajita A., Kita S., Osamura Y., Shinoda T. An autopsy case of diffuse myelomatosis associated with systemic kappa light chain deposition disease (LCDD). A patho-anatomical, immunohistochemical and immunobiochemical study. Acta Pathol Jpn. 1988 Apr;38(4):479–488. doi: 10.1111/j.1440-1827.1988.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Yang G. C., Nieto R., Stachura I., Gallo G. R. Ultrastructural immunohistochemical localization of polyclonal IgG, C3, and amyloid P component on the congo red-negative amyloid-like fibrils of fibrillary glomerulopathy. Am J Pathol. 1992 Aug;141(2):409–419. [PMC free article] [PubMed] [Google Scholar]