Abstract
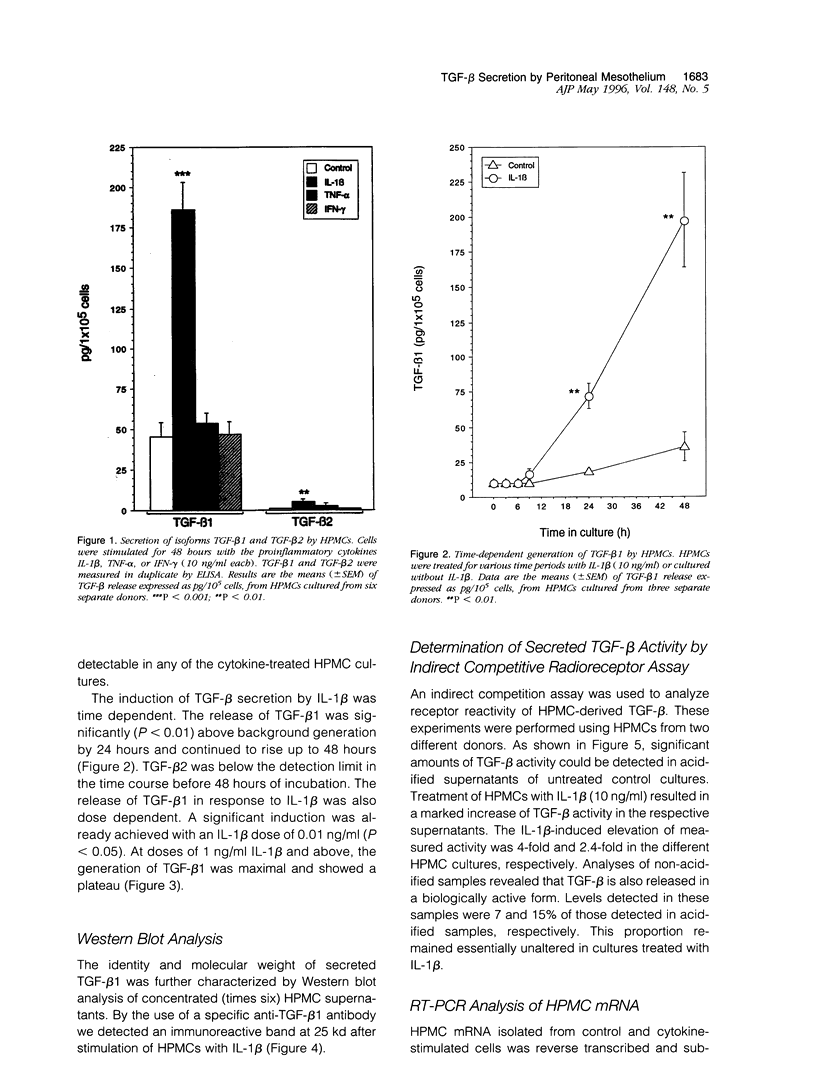
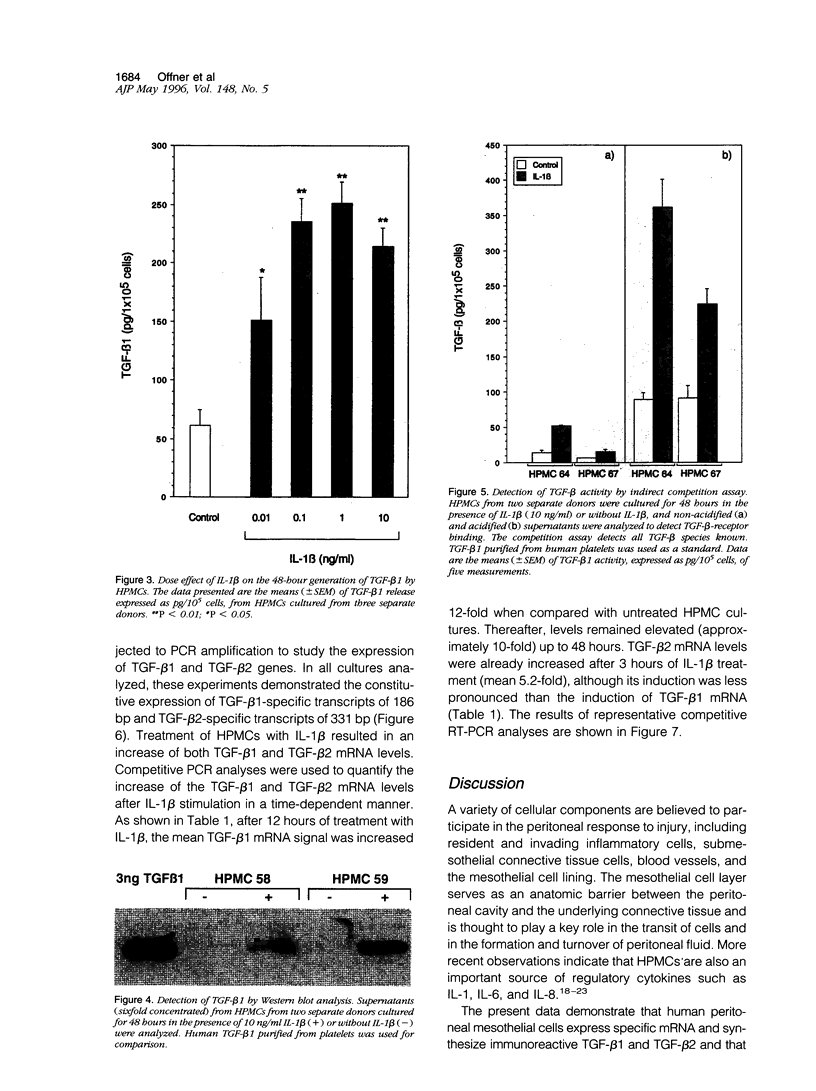
Peritoneal mesothelial cells are uniquely located to regulate cellular events in the peritoneal cavity and are a potentially important source for various cytokines. The present study was designed to elucidate the capacity of human peritoneal mesothelial cells (HPMCs) to synthesize and secrete the transforming growth factor (TGF)-beta isoforms 1, 2, and 3 and to characterize their regulation by inflammatory cytokines. HPMCs constitutively released appreciable amounts of TGF-beta 1 and low amounts of TGF-beta 2 as detected by specific immunoassays. TGF-beta 1 levels secreted within 48 hours (45 +/- 8.9 pg/10(5) cells) were 60-fold higher than TGF-beta 2 levels (0.9 +/- 0.1 pg/10(5) cells), respectively. Treatment of HPMCs with interleukin (IL)-1 beta (10 ng/ml) resulted in a significant increase of both TGF-beta 1 (mean, 5-fold; P < 0.001) and TGF-beta 2 (mean, 6-fold; P < 0.01) generation. After 48 hours of IL-1 beta treatment the levels were 185 +/- 17.1 pg/10(5) cells for TGF-beta 1 and 5.3 +/- 1.5 pg/10(5) cells for TGF-beta 2, respectively. Neither tumor necrosis factor (TNF)-alpha nor interferon (IFN)-gamma (both 10 ng/ml) affected TGF-beta 1 or TGF-beta 2 synthesis by HPMCs. TGF-beta 3 could not be detected in any of the supernatant media. Stimulation of HPMCs with IL-1 beta increased steady-state levels of TGF-beta 1- and TGF-beta 2-specific mRNA. Western blot analysis of supernatants revealed the presence of an immunoreactive band at 25 kd. Indirect competition assays confirmed receptor-binding activity of HPMC-derived TGF-beta. Appreciable amounts of TGF-beta were present in a bioactive form. Our results demonstrate that HPMCs synthesize the TGF-beta isoforms 1 and 2 and that the levels of mRNA and protein release can be up-regulated by the proinflammatory cytokine IL-1 beta.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrow M. G., Moses H. L. Transforming growth factor beta and cell cycle regulation. Cancer Res. 1995 Apr 1;55(7):1452–1457. [PubMed] [Google Scholar]
- Chegini N., Gold L. I., Williams R. S., Masterson B. J. Localization of transforming growth factor beta isoforms TGF-beta 1, TGF-beta 2, and TGF-beta 3 in surgically induced pelvic adhesions in the rat. Obstet Gynecol. 1994 Mar;83(3):449–454. [PubMed] [Google Scholar]
- Davila R. M., Crouch E. C. Role of mesothelial and submesothelial stromal cells in matrix remodeling following pleural injury. Am J Pathol. 1993 Feb;142(2):547–555. [PMC free article] [PubMed] [Google Scholar]
- Douvdevani A., Rapoport J., Konforty A., Argov S., Ovnat A., Chaimovitz C. Human peritoneal mesothelial cells synthesize IL-1 alpha and beta. Kidney Int. 1994 Oct;46(4):993–1001. doi: 10.1038/ki.1994.359. [DOI] [PubMed] [Google Scholar]
- Dubois C. M., Ruscetti F. W., Palaszynski E. W., Falk L. A., Oppenheim J. J., Keller J. R. Transforming growth factor beta is a potent inhibitor of interleukin 1 (IL-1) receptor expression: proposed mechanism of inhibition of IL-1 action. J Exp Med. 1990 Sep 1;172(3):737–744. doi: 10.1084/jem.172.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen A., Duclos B., Schmitt-Goguel M., Rouyer N., Bergerat J. P., Hummel M., Oskam R., Oberling F. Human resident peritoneal macrophages: phenotype and biology. Br J Haematol. 1994 Dec;88(4):712–722. doi: 10.1111/j.1365-2141.1994.tb05109.x. [DOI] [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Shalaby M. R., Lackides G. A., Lewis G. D., Shepard H. M., Palladino M. A., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987 Aug 1;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. R., Vadas M. A. Endothelial adhesiveness for blood neutrophils is inhibited by transforming growth factor-beta. Science. 1988 Oct 7;242(4875):97–99. doi: 10.1126/science.3175638. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Lechner J. F., Reddel R. R., Roberts A. B., Robbins K. C., Gabrielson E. W., Harris C. C. Comparison of production of transforming growth factor-beta and platelet-derived growth factor by normal human mesothelial cells and mesothelioma cell lines. Cancer Res. 1987 Dec 1;47(23):6180–6184. [PubMed] [Google Scholar]
- Hirte H. W., Clark D. A., O'Connell G., Rusthoven J., Mazurka J. Reversal of suppression of lymphokine-activated killer cells by transforming growth factor-beta in ovarian carcinoma ascitic fluid requires interleukin-2 combined with anti-CD3 antibody. Cell Immunol. 1992 Jun;142(1):207–216. doi: 10.1016/0008-8749(92)90281-s. [DOI] [PubMed] [Google Scholar]
- Hirte H., Clark D. A. Generation of lymphokine-activated killer cells in human ovarian carcinoma ascitic fluid: identification of transforming growth factor-beta as a suppressive factor. Cancer Immunol Immunother. 1991;32(5):296–302. doi: 10.1007/BF01789047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurteau J., Rodriguez G. C., Whitaker R. S., Shah S., Mills G., Bast R. C., Berchuck A. Transforming growth factor-beta inhibits proliferation of human ovarian cancer cells obtained from ascites. Cancer. 1994 Jul 1;74(1):93–99. doi: 10.1002/1097-0142(19940701)74:1<93::aid-cncr2820740117>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Jonjić N., Peri G., Bernasconi S., Sciacca F. L., Colotta F., Pelicci P., Lanfrancone L., Mantovani A. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med. 1992 Oct 1;176(4):1165–1174. doi: 10.1084/jem.176.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Ward J. M., Yaswen L., Mackall C. L., Bauer S. R., Huh C. G., Gress R. E., Karlsson S. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am J Pathol. 1995 Jan;146(1):264–275. [PMC free article] [PubMed] [Google Scholar]
- Kutteh W. H., Kutteh C. C. Quantitation of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in the effusions of ovarian epithelial neoplasms. Am J Obstet Gynecol. 1992 Dec;167(6):1864–1869. doi: 10.1016/0002-9378(92)91788-c. [DOI] [PubMed] [Google Scholar]
- Li B. Y., Mohanraj D., Olson M. C., Moradi M., Twiggs L., Carson L. F., Ramakrishnan S. Human ovarian epithelial cancer cells cultures in vitro express both interleukin 1 alpha and beta genes. Cancer Res. 1992 Apr 15;52(8):2248–2252. [PubMed] [Google Scholar]
- Maeda J., Ueki N., Ohkawa T., Iwahashi N., Nakano T., Hada T., Higashino K. Local production and localization of transforming growth factor-beta in tuberculous pleurisy. Clin Exp Immunol. 1993 Apr;92(1):32–38. doi: 10.1111/j.1365-2249.1993.tb05944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth C., Lang T., Koza A., Mayer I., Daxenbichler G. Transforming growth factor-beta and ovarian carcinoma cells: regulation of proliferation and surface antigen expression. Cancer Lett. 1990 Jun 15;51(3):221–225. doi: 10.1016/0304-3835(90)90106-8. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N. L., Wahl S. M. Transforming growth factor beta: a matter of life and death. J Leukoc Biol. 1994 Mar;55(3):401–409. doi: 10.1002/jlb.55.3.401. [DOI] [PubMed] [Google Scholar]
- Mukai M., Shinkai K., Komatsu K., Akedo H. Potentiation of invasive capacity of rat ascites hepatoma cells by transforming growth factor-beta. Jpn J Cancer Res. 1989 Feb;80(2):107–110. doi: 10.1111/j.1349-7006.1989.tb02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M., Sironi M., Polentarutti N., Mantovani A., Colotta F. Induction by transforming growth factor-beta 1 of the interleukin-1 receptor antagonist and of its intracellular form in human polymorphonuclear cells. Eur J Immunol. 1994 Dec;24(12):3194–3198. doi: 10.1002/eji.1830241242. [DOI] [PubMed] [Google Scholar]
- Offner F. A., Obrist P., Stadlmann S., Feichtinger H., Klingler P., Herold M., Zwierzina H., Hittmair A., Mikuz G., Abendstein B. IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine. 1995 Aug;7(6):542–547. doi: 10.1006/cyto.1995.0073. [DOI] [PubMed] [Google Scholar]
- Offner F. A., Wirtz H. C., Schiefer J., Bigalke I., Klosterhalfen B., Bittinger F., Mittermayer C., Kirkpatrick C. J. Interaction of human malignant melanoma (ST-ML-12) tumor spheroids with endothelial cell monolayers. Damage to endothelium by oxygen-derived free radicals. Am J Pathol. 1992 Sep;141(3):601–610. [PMC free article] [PubMed] [Google Scholar]
- Propst T., Propst A., Herold M., Schauer G., Judmaier G., Braunsteiner H., Stöffler G., Vogel W. Spontaneous bacterial peritonitis is associated with high levels of interleukin-6 and its secondary mediators in ascitic fluid. Eur J Clin Invest. 1993 Dec;23(12):832–836. doi: 10.1111/j.1365-2362.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Figari I. S., Espevik T., Palladino M. A., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987 Oct 1;166(4):991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeck U., Bossler A., Graeven U., Fox F. E., Nowell P. C., Knabbe C., Kari C. Transforming growth factor beta production and responsiveness in normal human melanocytes and melanoma cells. Cancer Res. 1994 Jan 15;54(2):575–581. [PubMed] [Google Scholar]
- Sanderson N., Factor V., Nagy P., Kopp J., Kondaiah P., Wakefield L., Roberts A. B., Sporn M. B., Thorgeirsson S. S. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambia G., Testa U., Panici P. B., Martucci R., Foti E., Petrini M., Amoroso M., Masciullo V., Peschle C., Mancuso S. Interleukin-6 serum levels in patients with gynecological tumors. Int J Cancer. 1994 May 1;57(3):318–323. doi: 10.1002/ijc.2910570305. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Ohzeki S., Utsumi K., Takiuchi H., Muramatsu M., Li X. F., Shimizu J., Fujiwara H., Hamaoka T. Transforming growth factor-beta-induced inhibition of T cell function. Susceptibility difference in T cells of various phenotypes and functions and its relevance to immunosuppression in the tumor-bearing state. J Immunol. 1991 Feb 1;146(3):1077–1082. [PubMed] [Google Scholar]
- Topley N., Brown Z., Jörres A., Westwick J., Davies M., Coles G. A., Williams J. D. Human peritoneal mesothelial cells synthesize interleukin-8. Synergistic induction by interleukin-1 beta and tumor necrosis factor-alpha. Am J Pathol. 1993 Jun;142(6):1876–1886. [PMC free article] [PubMed] [Google Scholar]
- Topley N., Jörres A., Luttmann W., Petersen M. M., Lang M. J., Thierauch K. H., Müller C., Coles G. A., Davies M., Williams J. D. Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1 beta and TNF alpha. Kidney Int. 1993 Jan;43(1):226–233. doi: 10.1038/ki.1993.36. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988 Jul 21;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Turner M., Chantry D., Katsikis P., Berger A., Brennan F. M., Feldmann M. Induction of the interleukin 1 receptor antagonist protein by transforming growth factor-beta. Eur J Immunol. 1991 Jul;21(7):1635–1639. doi: 10.1002/eji.1830210708. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Yin S., Guo K., Yi E. S., Remick D., del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am J Pathol. 1991 May;138(5):1097–1101. [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Welch D. R., Fabra A., Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S., Rossi A. M., Chegini N., Schultz G. Effect of transforming growth factor beta on postoperative adhesion formation and intact peritoneum. J Surg Res. 1992 Jan;52(1):65–70. doi: 10.1016/0022-4804(92)90280-d. [DOI] [PubMed] [Google Scholar]
- Wilson A. P., Fox H., Scott I. V., Lee H., Dent M., Golding P. R. A comparison of the growth promoting properties of ascitic fluids, cyst fluids and peritoneal fluids from patients with ovarian tumours. Br J Cancer. 1991 Jan;63(1):102–108. doi: 10.1038/bjc.1991.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Boyer C. M., Whitaker R. S., Berchuck A., Wiener J. R., Weinberg J. B., Bast R. C., Jr Tumor necrosis factor alpha as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor alpha expression. Cancer Res. 1993 Apr 15;53(8):1939–1944. [PubMed] [Google Scholar]
- Yanagihara K., Tsumuraya M. Transforming growth factor beta 1 induces apoptotic cell death in cultured human gastric carcinoma cells. Cancer Res. 1992 Jul 15;52(14):4042–4045. [PubMed] [Google Scholar]
- Yung S., Thomas G. J., Stylianou E., Williams J. D., Coles G. A., Davies M. Source of peritoneal proteoglycans. Human peritoneal mesothelial cells synthesize and secrete mainly small dermatan sulfate proteoglycans. Am J Pathol. 1995 Feb;146(2):520–529. [PMC free article] [PubMed] [Google Scholar]
- Zeillemaker A. M., Mul F. P., Hoynck van Papendrecht A. A., Kuijpers T. W., Roos D., Leguit P., Verbrugh H. A. Polarized secretion of interleukin-8 by human mesothelial cells: a role in neutrophil migration. Immunology. 1995 Feb;84(2):227–232. [PMC free article] [PubMed] [Google Scholar]
- de Martin R., Haendler B., Hofer-Warbinek R., Gaugitsch H., Wrann M., Schlüsener H., Seifert J. M., Bodmer S., Fontana A., Hofer E. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987 Dec 1;6(12):3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]