Abstract
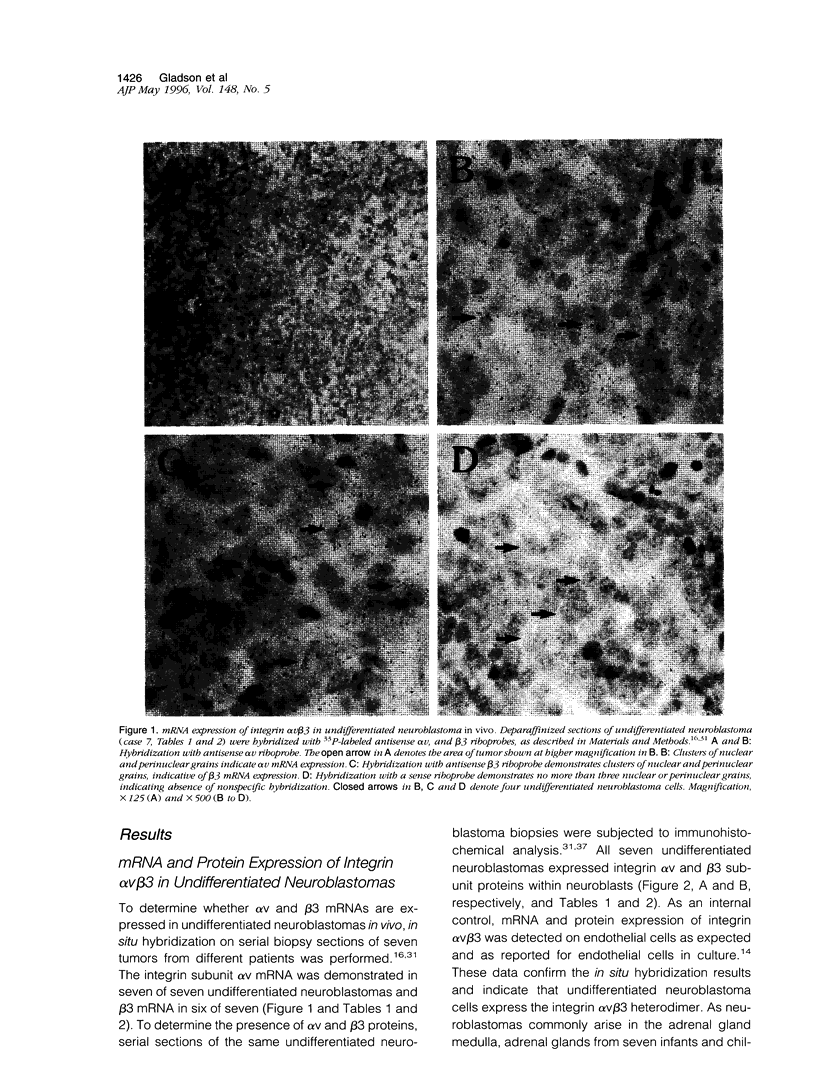
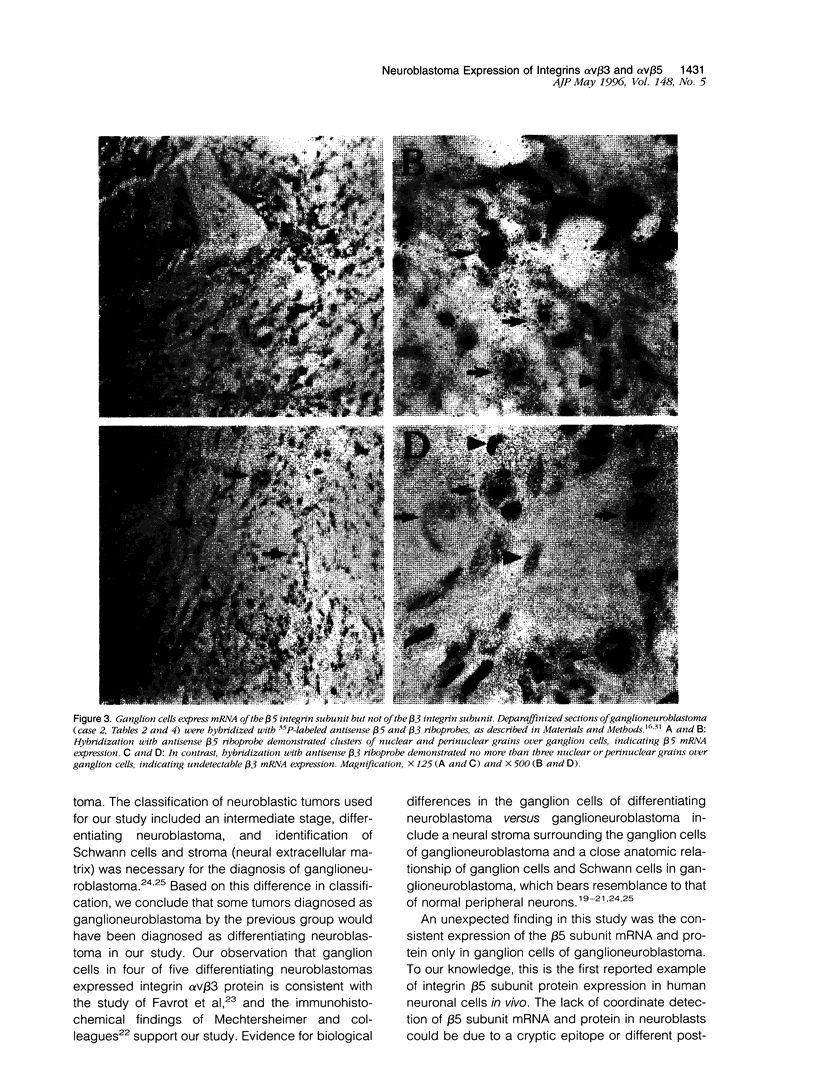
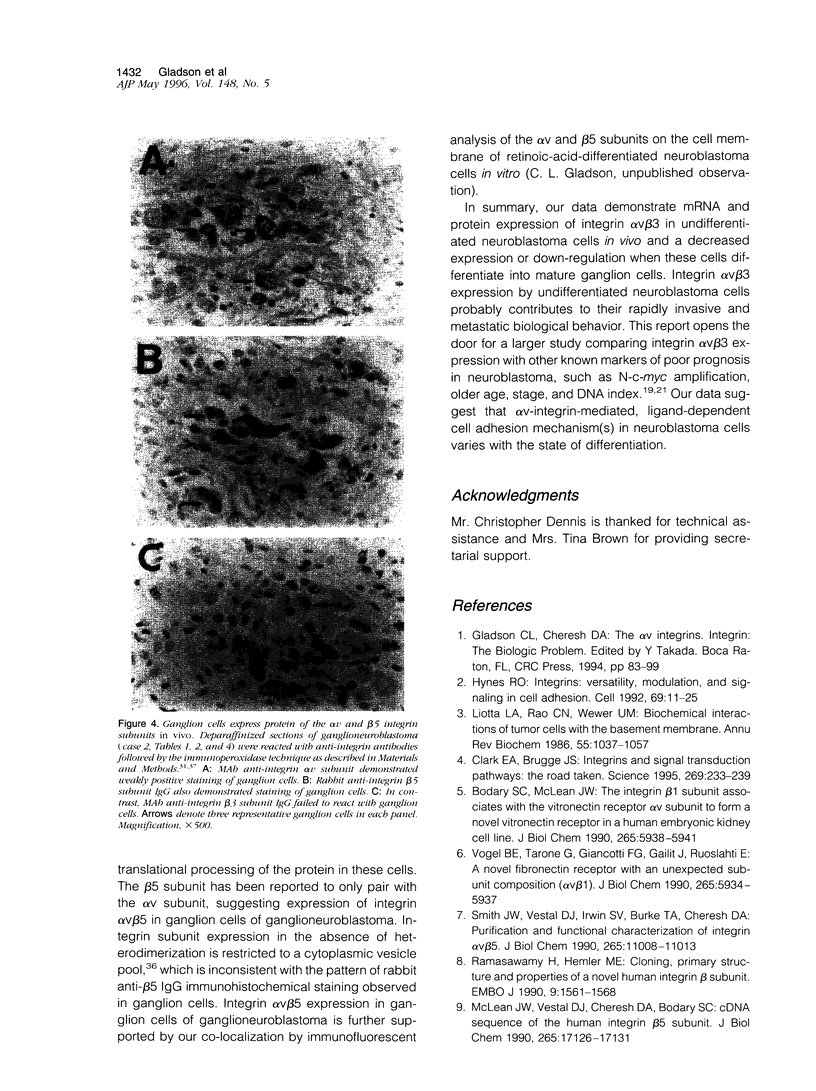
The ligand specificity of the integrin cell adhesion receptors probably determines the ability of specific integrins to promote tumor cell proliferation and metastasis. Therefore, we compared the expression of integrin alphaVbeta3, a promiscuous receptor that binds with high affinity to numerous cell matrix proteins, with the expression of integrin alphaVbeta5 and the integrin beta 1 subunit (which pairs with multiple alpha subunits) in neuroblastic tumors at various stages of differentiation. Undifferentiated neuroblastoma tumors rapidly invade and metastasize, whereas ganglioneuroblastomas rarely metastasize. Differentiating neuroblastomas are associated with an intermediate prognosis. Paraffin sections of neuroblastic tumors at various stages of differentiation obtained at biopsy from 17 patients were hybridized with antisense integrin subunit-specific alphaV, beta3, beta1, and beta5 riboprobes. All neuroblastic tumors and seven adrenal glands obtained at autopsy were analyzed immunohistochemically with antibodies directed toward the alphaV, beta3, beta1, and beta5 subunits. The alphaV subunit was expressed in neuroblastic tumors independent of the stage of differentiation, although mRNA and protein expression were generally weak in ganglioneuroblastomas, and was also detected in adrenal gland medullae. The beta1 subunit was detected in most neuroblastic tumors independent of the stage of differentiation as well as in adrenal gland medullae. In contrast, the beta3 subunit, which was not expressed in adrenal gland medullae, was expressed at the protein and mRNA levels in undifferentiated neuroblastomas (six of seven and seven of seven, respectively) but was not expressed in neuroblasts or ganglion cells in ganglioneuroblastomas (one case weakly positive out of five). The beta 5 subunit was expressed at the protein (five of five) and mRNA (four of five) levels in the ganglion cells of ganglioneuroblastomas and, although mRNA for this subunit was detectable in undifferentiated tumors, the protein was not detectable. The expression of integrin alphaVbeta3 in undifferentiated neuroblastomas may contribute to the rapid growth of these tumors and their tendency to metastasize.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Argraves W. S., Suzuki S., Arai H., Thompson K., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1183–1190. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodary S. C., McLean J. W. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990 Apr 15;265(11):5938–5941. [PubMed] [Google Scholar]
- Breuss J. M., Gallo J., DeLisser H. M., Klimanskaya I. V., Folkesson H. G., Pittet J. F., Nishimura S. L., Aldape K., Landers D. V., Carpenter W. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995 Jun;108(Pt 6):2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M., Stern C. D., Fraser S. Analysis of neural crest cell lineage and migration. J Craniofac Genet Dev Biol. 1991 Oct-Dec;11(4):214–222. [PubMed] [Google Scholar]
- Busk M., Pytela R., Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem. 1992 Mar 25;267(9):5790–5796. [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Clark E. A., Brugge J. S. Integrins and signal transduction pathways: the road taken. Science. 1995 Apr 14;268(5208):233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Favrot M. C., Combaret V., Goillot E., Lutz P., Frappaz D., Thiesse P., Thyss A., Dolbeau D., Bouffet E., Tabone E. Expression of integrin receptors on 45 clinical neuroblastoma specimens. Int J Cancer. 1991 Sep 30;49(3):347–355. doi: 10.1002/ijc.2910490306. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Steiner B., Rall S. C., Jr, Lo S. S., Phillips D. R. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to "integrin". J Biol Chem. 1987 Mar 25;262(9):3936–3939. [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Gladson C. L., Cheresh D. A. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991 Dec;88(6):1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladson C. L., Pijuan-Thompson V., Olman M. A., Gillespie G. Y., Yacoub I. Z. Up-regulation of urokinase and urokinase receptor genes in malignant astrocytoma. Am J Pathol. 1995 May;146(5):1150–1160. [PMC free article] [PubMed] [Google Scholar]
- Gladson C. L., Wilcox J. N., Sanders L., Gillespie G. Y., Cheresh D. A. Cerebral microenvironment influences expression of the vitronectin gene in astrocytic tumors. J Cell Sci. 1995 Mar;108(Pt 3):947–956. doi: 10.1242/jcs.108.3.947. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Liaw L., Skinner M. P., Raines E. W., Ross R., Cheresh D. A., Schwartz S. M., Giachelli C. M. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Invest. 1995 Feb;95(2):713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- McComb R. D., Bigner D. D. Immunolocalization of monoclonal antibody-defined extracellular matrix antigens in human brain tumors. J Neurooncol. 1985;3(2):181–186. doi: 10.1007/BF02228895. [DOI] [PubMed] [Google Scholar]
- McLean J. W., Vestal D. J., Cheresh D. A., Bodary S. C. cDNA sequence of the human integrin beta 5 subunit. J Biol Chem. 1990 Oct 5;265(28):17126–17131. [PubMed] [Google Scholar]
- Mechtersheimer G., Barth T., Quentmeier A., Möller P. Differential expression of beta 1, beta 3, and beta 4 integrin subunits in nonneoplastic neural cells of the peripheral and autonomic nervous system and in tumors derived from these cells. Lab Invest. 1994 May;70(5):740–752. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle M., Napier M. A., McLean J. W. Cloning and expression of a divergent integrin subunit beta 8. J Biol Chem. 1991 Oct 15;266(29):19650–19658. [PubMed] [Google Scholar]
- Newman P. J., Kahn R. A., Hines A. Detection and characterization of monoclonal antibodies to platelet membrane proteins. J Cell Biol. 1981 Jul;90(1):249–253. doi: 10.1083/jcb.90.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S. L., Sheppard D., Pytela R. Integrin alpha v beta 8. Interaction with vitronectin and functional divergence of the beta 8 cytoplasmic domain. J Biol Chem. 1994 Nov 18;269(46):28708–28715. [PubMed] [Google Scholar]
- Ramaswamy H., Hemler M. E. Cloning, primary structure and properties of a novel human integrin beta subunit. EMBO J. 1990 May;9(5):1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D., Rozzo C., Starr L., Quaranta V., Erle D. J., Pytela R. Complete amino acid sequence of a novel integrin beta subunit (beta 6) identified in epithelial cells using the polymerase chain reaction. J Biol Chem. 1990 Jul 15;265(20):11502–11507. [PubMed] [Google Scholar]
- Shimada H., Chatten J., Newton W. A., Jr, Sachs N., Hamoudi A. B., Chiba T., Marsden H. B., Misugi K. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984 Aug;73(2):405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Vestal D. J., Irwin S. V., Burke T. A., Cheresh D. A. Purification and functional characterization of integrin alpha v beta 5. An adhesion receptor for vitronectin. J Biol Chem. 1990 Jul 5;265(19):11008–11013. [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Arai H., Languino L. R., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the vitronectin receptor alpha subunit and comparative expression of adhesion receptor mRNAs. J Biol Chem. 1987 Oct 15;262(29):14080–14085. [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Pytela R., Arai H., Krusius T., Pierschbacher M. D., Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]