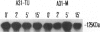Abstract
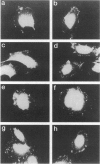
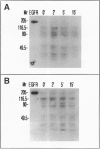
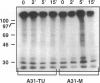
Autocrine motility factor (AMF) is a tumor-secreted cytokine that acts as a motogen as well as a mitogen via a receptor-mediated signaling pathway(s). Expression of the AMF receptor (AMF-R) in normal cells is regulated by cell contact whereas in transformed cells AMF-R is constitutively expressed irrespective of cell density. Here we have analyzed the regulation of AMF-R expression in a BALB/c angiosarcoma tumor system that allows investigation of cellular characteristics associated with tumor progression. The metastatic cell variant (A31-M) displayed a higher rate of unstimulated motility and responded to tumor-derived AMF locomotory stimulus as compared with the nonmetastatic cell variants (A31-TR and A31-TU) and, although a similar level of receptor expression was detected in cellular extracts from subconfluent cultures of these sublines, surface localization differed and cell contact down-regulated AMF-R expression in the normal but not the transformed cell counterparts. AMF promoted marked rearrangement of focal adhesion plaque proteins in the AMF migration-responsive cells exclusively. Reorganization of vinculin after AMF stimulation was paralleled by morphological redistribution of tyrosine-phosphorylated proteins and the tyrosine kinase pp125FAK in the migration-responsive cells; however, we did not observe a concomitant change in the pp125FAK phosphorylation state or the general level of cellular tyrosine phosphorylation in response to treatment, suggesting that the induction of cellular migration by AMF is independent of tyrosine phosphorylation events at the focal contacts and may therefore represent a novel pathway of cytokine-induced migration regulation.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benlimame N., Simard D., Nabi I. R. Autocrine motility factor receptor is a marker for a distinct membranous tubular organelle. J Cell Biol. 1995 Apr;129(2):459–471. doi: 10.1083/jcb.129.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt K. E., Graves L. M., Raines E. W., Igarashi Y., Wayman G., Yamamura S., Yatomi Y., Sidhu J. S., Krebs E. G., Hakomori S. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995 Jul;130(1):193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Doyle G. M., Sharief Y., Mohler J. L. Prediction of metastatic potential by cancer cell motility in the Dunning R-3327 prostatic adenocarcinoma in vivo model. J Urol. 1992 Feb;147(2):514–518. doi: 10.1016/s0022-5347(17)37291-9. [DOI] [PubMed] [Google Scholar]
- Erdel M., Spiess E., Trefz G., Boxberger H. J., Ebert W. Cell interactions and motility in human lung tumor cell lines HS-24 and SB-3 under the influence of extracellular matrix components and proteinase inhibitors. Anticancer Res. 1992 Mar-Apr;12(2):349–359. [PubMed] [Google Scholar]
- Erickson C. A. Cell migration in the embryo and adult organism. Curr Opin Cell Biol. 1990 Feb;2(1):67–74. doi: 10.1016/s0955-0674(05)80033-x. [DOI] [PubMed] [Google Scholar]
- Guirguis R., Margulies I., Taraboletti G., Schiffmann E., Liotta L. Cytokine-induced pseudopodial protrusion is coupled to tumour cell migration. Nature. 1987 Sep 17;329(6136):261–263. doi: 10.1038/329261a0. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Wang C., Lange L. A., Turley E. A. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J Cell Biol. 1994 Jul;126(2):575–588. doi: 10.1083/jcb.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann G., Weidner K. M., Schwarz H., Birchmeier W. The motility signal of scatter factor/hepatocyte growth factor mediated through the receptor tyrosine kinase met requires intracellular action of Ras. J Biol Chem. 1994 Sep 2;269(35):21936–21939. [PubMed] [Google Scholar]
- Hendrix M. J., Wood W. R., Seftor E. A., Lotan D., Nakajima M., Misiorowski R. L., Seftor R. E., Stetler-Stevenson W. G., Bevacqua S. J., Liotta L. A. Retinoic acid inhibition of human melanoma cell invasion through a reconstituted basement membrane and its relation to decreases in the expression of proteolytic enzymes and motility factor receptor. Cancer Res. 1990 Jul 1;50(13):4121–4130. [PubMed] [Google Scholar]
- Hildebrand J. D., Schaller M. D., Parsons J. T. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993 Nov;123(4):993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishisaki A., Oida S., Momose F., Amagasa T., Rikimaru K., Ichijo H., Sasaki S. Identification and characterization of autocrine-motility-factor-like activity in oral squamous-cell-carcinoma cells. Int J Cancer. 1994 Dec 15;59(6):783–788. doi: 10.1002/ijc.2910590613. [DOI] [PubMed] [Google Scholar]
- Kohn E. C., Liotta L. A., Schiffmann E. Autocrine motility factor stimulates a three-fold increase in inositol trisphosphate in human melanoma cells. Biochem Biophys Res Commun. 1990 Jan 30;166(2):757–764. doi: 10.1016/0006-291x(90)90874-m. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Stracke M. L., Aznavoorian S. A., Beckner M. E., Schiffmann E. Tumor cell motility. Semin Cancer Biol. 1991 Apr;2(2):111–114. [PubMed] [Google Scholar]
- Lotan R., Amos B., Watanabe H., Raz A. Suppression of melanoma cell motility factor receptor expression by retinoic acid. Cancer Res. 1992 Sep 15;52(18):4878–4884. [PubMed] [Google Scholar]
- McCarthy J. B., Furcht L. T. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol. 1984 Apr;98(4):1474–1480. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi I. R., Raz A. Cell shape modulation alters glycosylation of a metastatic melanoma cell-surface antigen. Int J Cancer. 1987 Sep 15;40(3):396–402. doi: 10.1002/ijc.2910400319. [DOI] [PubMed] [Google Scholar]
- Nabi I. R., Watanabe H., Raz A. Autocrine motility factor and its receptor: role in cell locomotion and metastasis. Cancer Metastasis Rev. 1992 Mar;11(1):5–20. doi: 10.1007/BF00047599. [DOI] [PubMed] [Google Scholar]
- Nabi I. R., Watanabe H., Raz A. Identification of B16-F1 melanoma autocrine motility-like factor receptor. Cancer Res. 1990 Jan 15;50(2):409–414. [PubMed] [Google Scholar]
- Nakamori S., Watanabe H., Kameyama M., Imaoka S., Furukawa H., Ishikawa O., Sasaki Y., Kabuto T., Raz A. Expression of autocrine motility factor receptor in colorectal cancer as a predictor for disease recurrence. Cancer. 1994 Oct 1;74(7):1855–1862. doi: 10.1002/1097-0142(19941001)74:7<1855::aid-cncr2820740705>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Otto T., Birchmeier W., Schmidt U., Hinke A., Schipper J., Rübben H., Raz A. Inverse relation of E-cadherin and autocrine motility factor receptor expression as a prognostic factor in patients with bladder carcinomas. Cancer Res. 1994 Jun 15;54(12):3120–3123. [PubMed] [Google Scholar]
- Parsons J. T., Weber M. J. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr Top Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- Raz A., Geiger B. Altered organization of cell-substrate contacts and membrane-associated cytoskeleton in tumor cell variants exhibiting different metastatic capabilities. Cancer Res. 1982 Dec;42(12):5183–5190. [PubMed] [Google Scholar]
- Rodríguez Fernández J. L., Geiger B., Salomon D., Ben-Ze'ev A. Suppression of vinculin expression by antisense transfection confers changes in cell morphology, motility, and anchorage-dependent growth of 3T3 cells. J Cell Biol. 1993 Sep;122(6):1285–1294. doi: 10.1083/jcb.122.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer L. H., Burridge K., Turner C. E. Signaling between the extracellular matrix and the cytoskeleton: tyrosine phosphorylation and focal adhesion assembly. Cold Spring Harb Symp Quant Biol. 1992;57:193–202. doi: 10.1101/sqb.1992.057.01.024. [DOI] [PubMed] [Google Scholar]
- Samuels M., Ezzell R. M., Cardozo T. J., Critchley D. R., Coll J. L., Adamson E. D. Expression of chicken vinculin complements the adhesion-defective phenotype of a mutant mouse F9 embryonal carcinoma cell. J Cell Biol. 1993 May;121(4):909–921. doi: 10.1083/jcb.121.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silletti S., Raz A. Autocrine motility factor is a growth factor. Biochem Biophys Res Commun. 1993 Jul 15;194(1):446–457. doi: 10.1006/bbrc.1993.1840. [DOI] [PubMed] [Google Scholar]
- Silletti S., Timar J., Honn K. V., Raz A. Autocrine motility factor induces differential 12-lipoxygenase expression and activity in high- and low-metastatic K1735 melanoma cell variants. Cancer Res. 1994 Nov 15;54(22):5752–5756. [PubMed] [Google Scholar]
- Silletti S., Watanabe H., Hogan V., Nabi I. R., Raz A. Purification of B16-F1 melanoma autocrine motility factor and its receptor. Cancer Res. 1991 Jul 1;51(13):3507–3511. [PubMed] [Google Scholar]
- Silletti S., Yao J. P., Pienta K. J., Raz A. Loss of cell-contact regulation and altered responses to autocrine motility factor correlate with increased malignancy in prostate cancer cells. Int J Cancer. 1995 Sep 27;63(1):100–105. doi: 10.1002/ijc.2910630118. [DOI] [PubMed] [Google Scholar]
- Stoker M., Gherardi E. Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta. 1991 Apr 16;1072(1):81–102. doi: 10.1016/0304-419x(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Stracke M. L., Guirguis R., Liotta L. A., Schiffmann E. Pertussis toxin inhibits stimulated motility independently of the adenylate cyclase pathway in human melanoma cells. Biochem Biophys Res Commun. 1987 Jul 15;146(1):339–345. doi: 10.1016/0006-291x(87)90730-3. [DOI] [PubMed] [Google Scholar]
- Timar J., Silletti S., Bazaz R., Raz A., Honn K. V. Regulation of melanoma-cell motility by the lipoxygenase metabolite 12-(S)-HETE. Int J Cancer. 1993 Dec 2;55(6):1003–1010. doi: 10.1002/ijc.2910550621. [DOI] [PubMed] [Google Scholar]
- Van Roy F., Mareel M. Tumour invasion: effects of cell adhesion and motility. Trends Cell Biol. 1992 Jun;2(6):163–169. doi: 10.1016/0962-8924(92)90035-l. [DOI] [PubMed] [Google Scholar]
- Volk T., Geiger B., Raz A. Motility and adhesive properties of high- and low-metastatic murine neoplastic cells. Cancer Res. 1984 Feb;44(2):811–824. [PubMed] [Google Scholar]
- Watanabe H., Carmi P., Hogan V., Raz T., Silletti S., Nabi I. R., Raz A. Purification of human tumor cell autocrine motility factor and molecular cloning of its receptor. J Biol Chem. 1991 Jul 15;266(20):13442–13448. [PubMed] [Google Scholar]
- Zvibel I., Raz A. The establishment and characterization of a new BALB/c angiosarcoma tumor system. Int J Cancer. 1985 Aug 15;36(2):261–272. doi: 10.1002/ijc.2910360220. [DOI] [PubMed] [Google Scholar]