Abstract
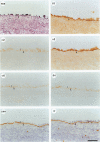
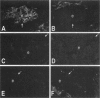
The surface of a normal ovary is covered by a monolayer of epithelial cells that rest on a basement membrane. The glycoprotein laminin is the major noncollagenous protein present in the basement membrane. The integrins alpha 1 beta 1, alpha 2 beta 1, alpha 3 beta 1, alpha 6 beta 1, and alpha 6 beta 4 serve as cell surface receptors for laminin. During the progression of serous ovarian carcinoma, tumor cells are frequently exfoliated from the surface of the ovary, thereby losing contact with the basement membrane. This study was designed to determine whether alterations in integrin expression may be associated with the malignant phenotype of the primary ovarian tumor and exfoliated ovarian carcinoma cells in the ascites fluid. By immunohistochemical staining, the entire surface of epithelial cells of normal ovaries stained positively for beta 1, alpha 2, and alpha 3 integrins, whereas only the basal surface of the epithelial cells, where they are in contact with laminin, stained positively for alpha 6 and beta 4. The entire surface of epithelial cells of solid tumors from patients with serous ovarian carcinoma stained positively for beta 1, alpha 2, and alpha 3 integrins. In most cases, no intact basement membrane surrounded the tumor nests, and staining for alpha 6 and beta 4 was irregular. When present, the basement membrane stained positively for laminin, and the basal surface of the epithelial cells stained positively for alpha 6 and beta 4. Ovarian carcinoma ascites cells exhibited a distinct phenotype, with a significant decrease in expression of the alpha 6 and beta 4 integrin subunits. As alpha 6 and beta 4 integrin subunits are present at the basal surface of many epithelial cells and serve as receptors for laminin, it is possible that ovarian carcinoma epithelial cells may be released from the basement membrane of the ovary due to their deficit of alpha 6 and beta 4 integrin subunits.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartolazzi A., Kaczmarek J., Nicoló G., Risso A. M., Tarone G., Rossino P., Defilippi P., Castellani P. Localization of the alpha 3 beta 1 integrin in some common epithelial tumors of the ovary and in normal equivalents. Anticancer Res. 1993 Jan-Feb;13(1):1–11. [PubMed] [Google Scholar]
- Bottini C., Miotti S., Fiorucci S., Facheris P., Ménard S., Colnaghi M. I. Polarization of the alpha 6 beta 4 integrin in ovarian carcinomas. Int J Cancer. 1993 May 8;54(2):261–267. doi: 10.1002/ijc.2910540217. [DOI] [PubMed] [Google Scholar]
- Bridges J.E., Englefield P., Boyd I.E., Roche W.R., Thomas E.J. Expression of integrin adhesion molecules in normal ovary and epithelial ovarian tumors. Int J Gynecol Cancer. 1995 May;5(3):187–192. doi: 10.1046/j.1525-1438.1995.05030187.x. [DOI] [PubMed] [Google Scholar]
- Campo E., Merino M. J., Tavassoli F. A., Charonis A. S., Stetler-Stevenson W. G., Liotta L. A. Evaluation of basement membrane components and the 72 kDa type IV collagenase in serous tumors of the ovary. Am J Surg Pathol. 1992 May;16(5):500–507. doi: 10.1097/00000478-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Cannistra S. A., Kansas G. S., Niloff J., DeFranzo B., Kim Y., Ottensmeier C. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993 Aug 15;53(16):3830–3838. [PubMed] [Google Scholar]
- Dedhar S., Saulnier R., Nagle R., Overall C. M. Specific alterations in the expression of alpha 3 beta 1 and alpha 6 beta 4 integrins in highly invasive and metastatic variants of human prostate carcinoma cells selected by in vitro invasion through reconstituted basement membrane. Clin Exp Metastasis. 1993 Sep;11(5):391–400. doi: 10.1007/BF00132982. [DOI] [PubMed] [Google Scholar]
- Eidelman S., Damsky C. H., Wheelock M. J., Damjanov I. Expression of the cell-cell adhesion glycoprotein cell-CAM 120/80 in normal human tissues and tumors. Am J Pathol. 1989 Jul;135(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Engel J. Laminins and other strange proteins. Biochemistry. 1992 Nov 10;31(44):10643–10651. doi: 10.1021/bi00159a001. [DOI] [PubMed] [Google Scholar]
- Fogh J., Wright W. C., Loveless J. D. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst. 1977 Feb;58(2):209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Coates P., Lemoine N. R., Horton M. A. Characterization of integrin chains in normal and neoplastic human pancreas. J Pathol. 1991 Sep;165(1):33–41. doi: 10.1002/path.1711650107. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Humphries M. J., Mould A. P., Tuckwell D. S. Dynamic aspects of adhesion receptor function--integrins both twist and shout. Bioessays. 1993 Jun;15(6):391–397. doi: 10.1002/bies.950150605. [DOI] [PubMed] [Google Scholar]
- Hurteau J., Rodriguez G. C., Whitaker R. S., Shah S., Mills G., Bast R. C., Berchuck A. Transforming growth factor-beta inhibits proliferation of human ovarian cancer cells obtained from ascites. Cancer. 1994 Jul 1;74(1):93–99. doi: 10.1002/1097-0142(19940701)74:1<93::aid-cncr2820740117>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Steinman H. K., Goldsmith B. A. Hemidesmosomes, collagen VII, and intermediate filaments in basal cell carcinoma. J Invest Dermatol. 1989 Nov;93(5):662–671. doi: 10.1111/1523-1747.ep12319833. [DOI] [PubMed] [Google Scholar]
- Kimmel K. A., Carey T. E. Altered expression in squamous carcinoma cells of an orientation restricted epithelial antigen detected by monoclonal antibody A9. Cancer Res. 1986 Jul;46(7):3614–3623. [PubMed] [Google Scholar]
- Kleinman H. K., Weeks B. S., Schnaper H. W., Kibbey M. C., Yamamura K., Grant D. S. The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases. Vitam Horm. 1993;47:161–186. doi: 10.1016/s0083-6729(08)60446-x. [DOI] [PubMed] [Google Scholar]
- Koukoulis G. K., Virtanen I., Korhonen M., Laitinen L., Quaranta V., Gould V. E. Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991 Oct;139(4):787–799. [PMC free article] [PubMed] [Google Scholar]
- Lee E. C., Lotz M. M., Steele G. D., Jr, Mercurio A. M. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992 May;117(3):671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau P. C., Wire J. P. Three-dimensional organization of stable microtubules and the Golgi apparatus in the somata of developing chick sensory neurons. J Neurocytol. 1995 Mar;24(3):207–223. doi: 10.1007/BF01181535. [DOI] [PubMed] [Google Scholar]
- Liebert M., Washington R., Wedemeyer G., Carey T. E., Grossman H. B. Loss of co-localization of alpha 6 beta 4 integrin and collagen VII in bladder cancer. Am J Pathol. 1994 Apr;144(4):787–795. [PMC free article] [PubMed] [Google Scholar]
- Mariani Costantini R., Falcioni R., Battista P., Zupi G., Kennel S. J., Colasante A., Venturo I., Curio C. G., Sacchi A. Integrin (alpha 6/beta 4) expression in human lung cancer as monitored by specific monoclonal antibodies. Cancer Res. 1990 Sep 15;50(18):6107–6112. [PubMed] [Google Scholar]
- Mercurio A. M. Laminin: multiple forms, multiple receptors. Curr Opin Cell Biol. 1990 Oct;2(5):845–849. doi: 10.1016/0955-0674(90)90082-p. [DOI] [PubMed] [Google Scholar]
- Pattaramalai S., Skubitz A. P. Promotion of human oral squamous cell carcinoma adhesion in vitro by the carboxy-terminal globular domain of laminin. Arch Oral Biol. 1994 Nov;39(11):925–933. doi: 10.1016/0003-9969(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Pattaramalai S., Skubitz K. M., Skubitz A. P. A novel recognition site on laminin for the alpha 3 beta 1 integrin. Exp Cell Res. 1996 Feb 1;222(2):281–290. doi: 10.1006/excr.1996.0036. [DOI] [PubMed] [Google Scholar]
- Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992;27(1-2):93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- Rossen K., Dahlstrøm K. K., Mercurio A. M., Wewer U. M. Expression of the alpha 6 beta 4 integrin by squamous cell carcinomas and basal cell carcinomas: possible relation to invasive potential? Acta Derm Venereol. 1994 Mar;74(2):101–105. doi: 10.2340/0001555574101105. [DOI] [PubMed] [Google Scholar]
- Schuger L., Varani J., Killen P. D., Skubitz A. P., Gilbride K. Laminin expression in the mouse lung increases with development and stimulates spontaneous organotypic rearrangement of mixed lung cells. Dev Dyn. 1992 Sep;195(1):43–54. doi: 10.1002/aja.1001950105. [DOI] [PubMed] [Google Scholar]
- See G., Briard J., Czernichow P. Fibrose du quadriceps consécutive à des injections intra-musculaires pratiqueés chez le prématuré et le nourrisson. Ann Pediatr (Paris) 1968 Feb 2;15(2):104–110. [PubMed] [Google Scholar]
- Shimoyama Y., Hirohashi S., Hirano S., Noguchi M., Shimosato Y., Takeichi M., Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989 Apr 15;49(8):2128–2133. [PubMed] [Google Scholar]
- Shiozaki H., Tahara H., Oka H., Miyata M., Kobayashi K., Tamura S., Iihara K., Doki Y., Hirano S., Takeichi M. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol. 1991 Jul;139(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Sollberg S., Peltonen J., Uitto J. Differential expression of laminin isoforms and beta 4 integrin epitopes in the basement membrane zone of normal human skin and basal cell carcinomas. J Invest Dermatol. 1992 Jun;98(6):864–870. doi: 10.1111/1523-1747.ep12457080. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Janssen H., Hogervorst F., Calafat J., Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987 Jul 25;262(21):10376–10383. [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Stallmach A., von Lampe B., Matthes H., Bornhöft G., Riecken E. O. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut. 1992 Mar;33(3):342–346. doi: 10.1136/gut.33.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch A. L., Carson L. F., Ramakrishnan S. Differential expression of the cell-cell adhesion molecule E-cadherin in ascites and solid human ovarian tumor cells. Int J Cancer. 1994 Aug 1;58(3):393–399. doi: 10.1002/ijc.2910580315. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Gil S. G., Murphy G. F., Wilke M. S., Carter W. G. Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for alpha 3 beta 1 positive T lymphocytes. J Cell Biol. 1993 Jun;121(5):1141–1152. doi: 10.1083/jcb.121.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M. S., Hsu B. M., Wille J. J., Jr, Pittelkow M. R., Scott R. E. Biologic mechanisms for the regulation of normal human keratinocyte proliferation and differentiation. Am J Pathol. 1988 Apr;131(1):171–181. [PMC free article] [PubMed] [Google Scholar]
- Wilke M. S., Skubitz A. P. Human keratinocytes adhere to multiple distinct peptide sequences of laminin. J Invest Dermatol. 1991 Jul;97(1):141–146. doi: 10.1111/1523-1747.ep12479311. [DOI] [PubMed] [Google Scholar]
- Wolf G. T., Carey T. E., Schmaltz S. P., McClatchey K. D., Poore J., Glaser L., Hayashida D. J., Hsu S. Altered antigen expression predicts outcome in squamous cell carcinoma of the head and neck. J Natl Cancer Inst. 1990 Oct 3;82(19):1566–1572. doi: 10.1093/jnci/82.19.1566. [DOI] [PubMed] [Google Scholar]