Abstract
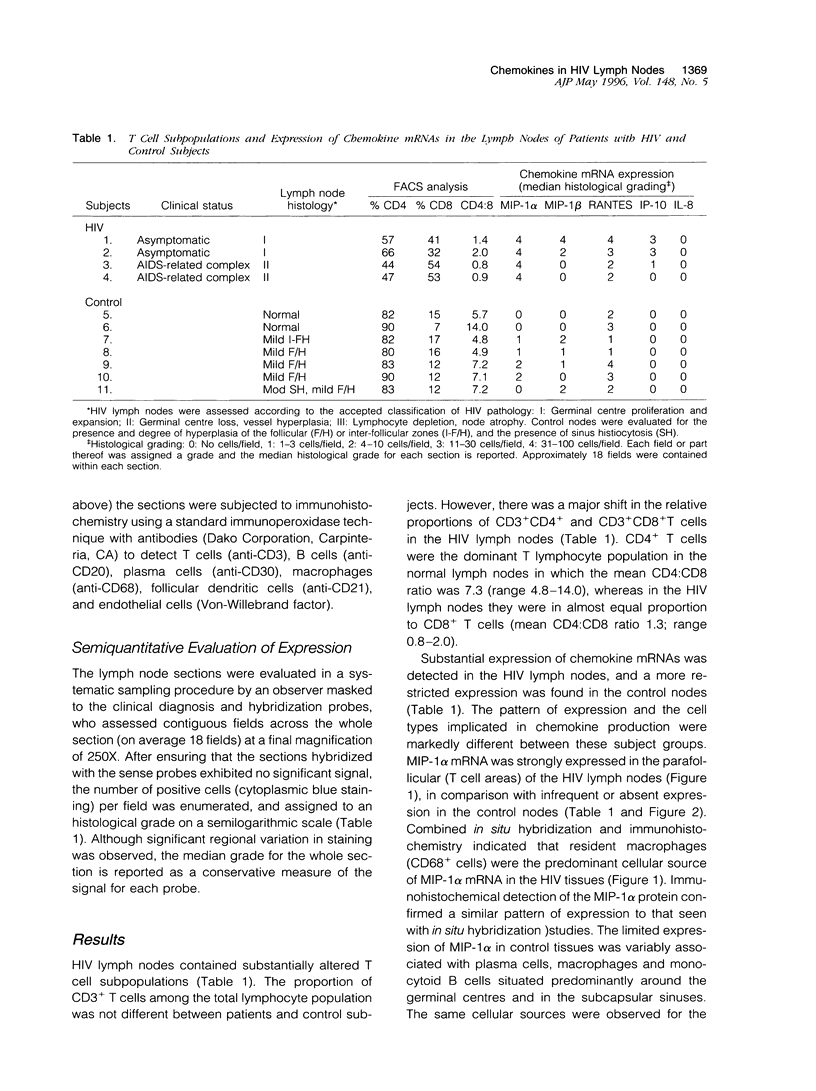
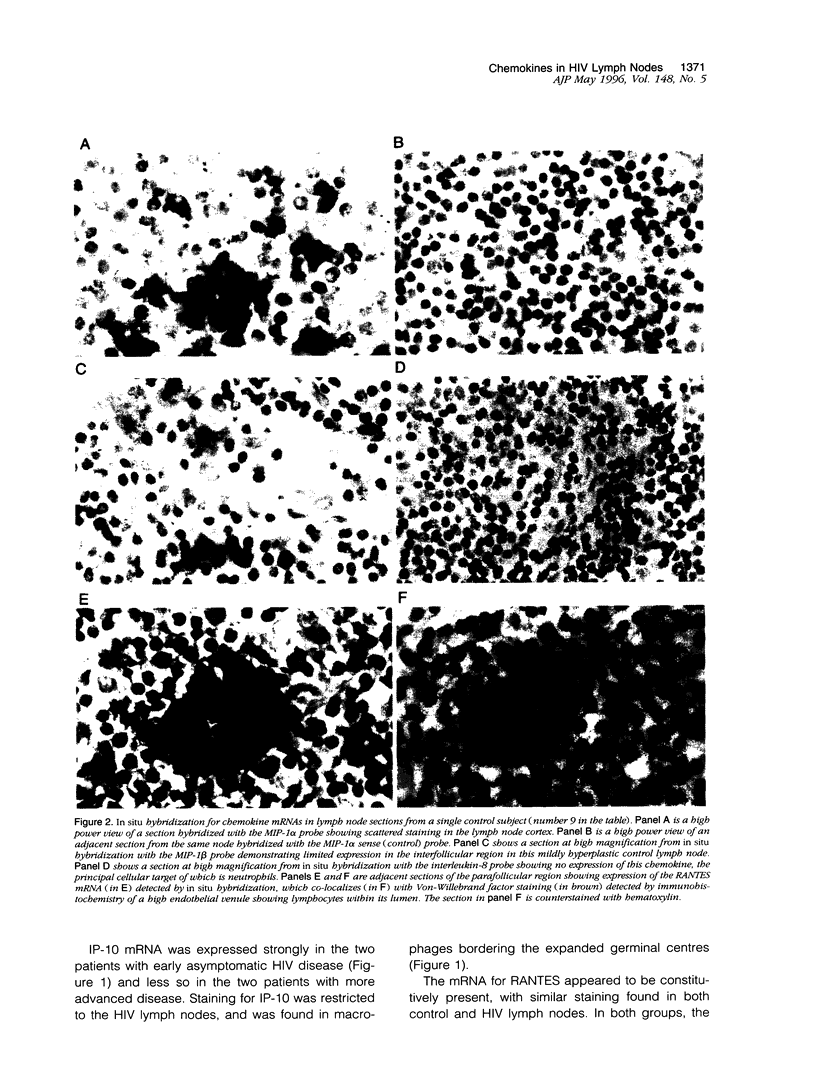
Recruitment of T lymphocytes to lymph nodes in patients with HIV infection is critical to the pathogenesis of disease. Chemokines are a family of cytokines, which are potent regulators of leukocyte migration. We studied the leukocyte populations and expression of chemokines known to be active upon T cells in lymph nodes of four HIV infected patients and seven control subjects using in situ hybridization, immunohistochemistry, and FACS analysis. The HIV lymph nodes showed CD8+ T lymphocyte accumulation and strongly enhanced chemokine expression, notably for the CD8+ T cell chemoattractant, macrophage inflammatory protein (MIP)-1 alpha. Resident macrophages appeared to be a major cellular source of chemokines in the HIV nodes. RANTES expression was present in both HIV and control lymph nodes, suggesting a physiological role for this chemokine in T lymphocyte recirculation. Chemokines may be important determinants of T lymphocyte accumulation in lymphoid tissue of patients with HIV/AIDS.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahill R. N., Frost H., Trnka Z. The effects of antigen on the migration of recirculating lymphocytes through single lymph nodes. J Exp Med. 1976 Apr 1;143(4):870–888. doi: 10.1084/jem.143.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994 Dec;15(12):575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Devergne O., Marfaing-Koka A., Schall T. J., Leger-Ravet M. B., Sadick M., Peuchmaur M., Crevon M. C., Kim K. J., Schall T. T., Kim T. Production of the RANTES chemokine in delayed-type hypersensitivity reactions: involvement of macrophages and endothelial cells. J Exp Med. 1994 May 1;179(5):1689–1694. doi: 10.1084/jem.179.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold J., Marche C., Audouin J., Aubert J. P., Le Tourneau A., Bouton C., Reynes M., Wizniak J., Capron F., Tricottet V. Lymph node modification in patients with the acquired immunodeficiency syndrome (AIDS) or with AIDS related complex (ARC). A histological, immuno-histopathological and ultrastructural study of 45 cases. Pathol Res Pract. 1985 Dec;180(6):590–611. doi: 10.1016/S0344-0338(85)80037-6. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Randolph G. J. Chemokines and tissue injury. Am J Pathol. 1995 Jun;146(6):1287–1301. [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. R., Oppenheim J. J., Kelvin D. J., Taub D. D. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996 Feb 1;156(3):932–938. [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Poli G., Fauci A. S. Cytokine modulation of HIV expression. Semin Immunol. 1993 Jun;5(3):165–173. doi: 10.1006/smim.1993.1020. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Camp R. D., Kaspari J. W., Goeddel D. V. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993 Jun 1;177(6):1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J. Biology of the RANTES/SIS cytokine family. Cytokine. 1991 May;3(3):165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- Schnizlein C. T., Kosco M. H., Szakal A. K., Tew J. G. Follicular dendritic cells in suspension: identification, enrichment, and initial characterization indicating immune complex trapping and lack of adherence and phagocytic activity. J Immunol. 1985 Mar;134(3):1360–1368. [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993 Jan 7;361(6407):79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Taub D. D., Conlon K., Lloyd A. R., Oppenheim J. J., Kelvin D. J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993 Apr 16;260(5106):355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Taub D. D., Lloyd A. R., Conlon K., Wang J. M., Ortaldo J. R., Harada A., Matsushima K., Kelvin D. J., Oppenheim J. J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993 Jun 1;177(6):1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]




