Abstract
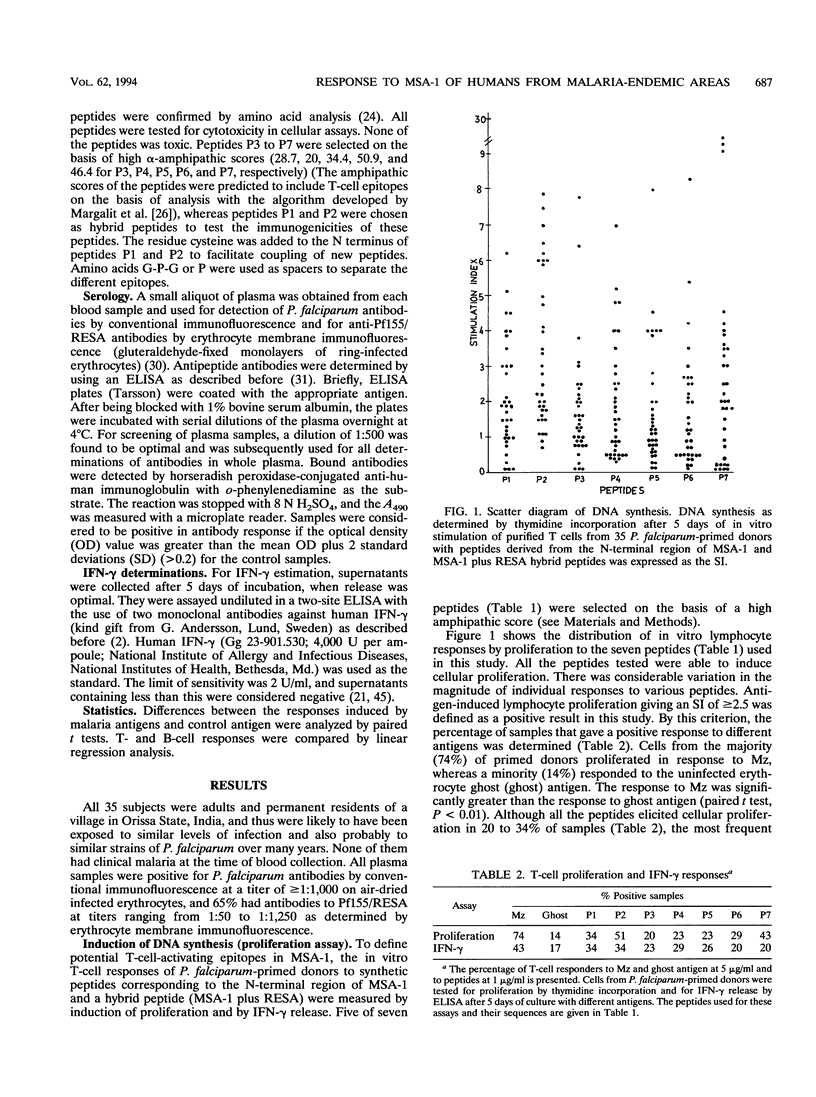
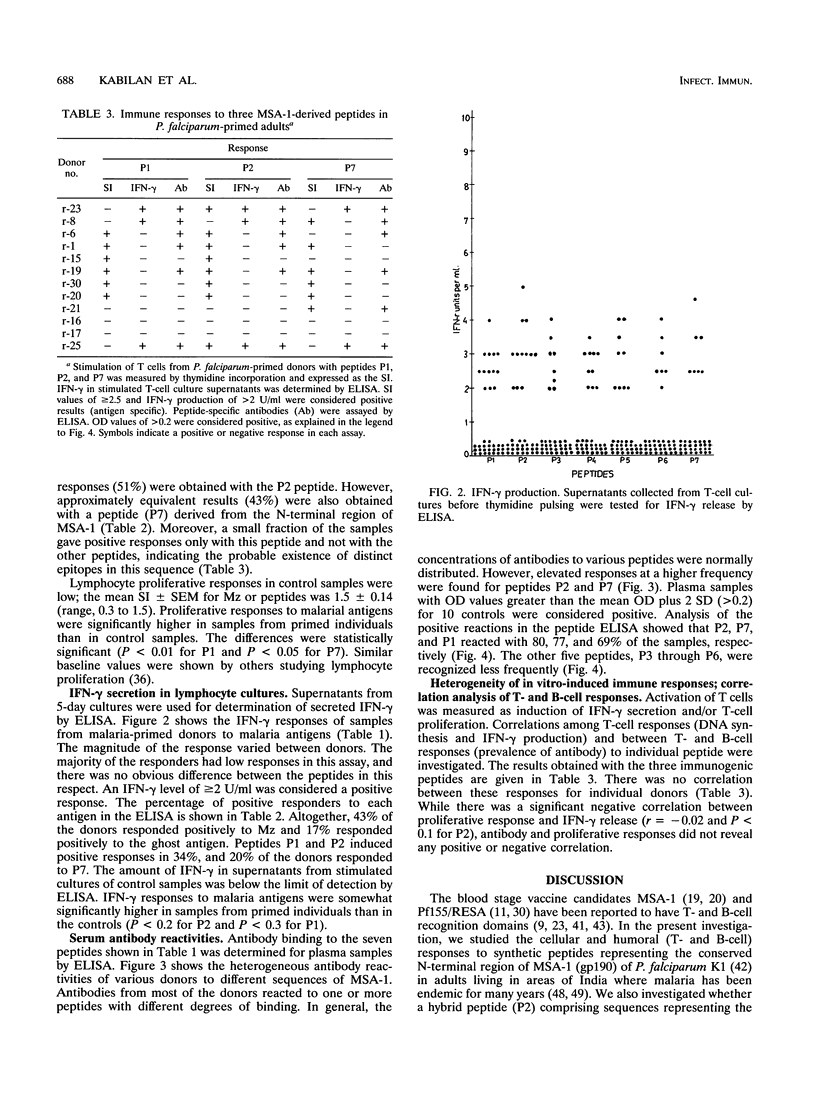
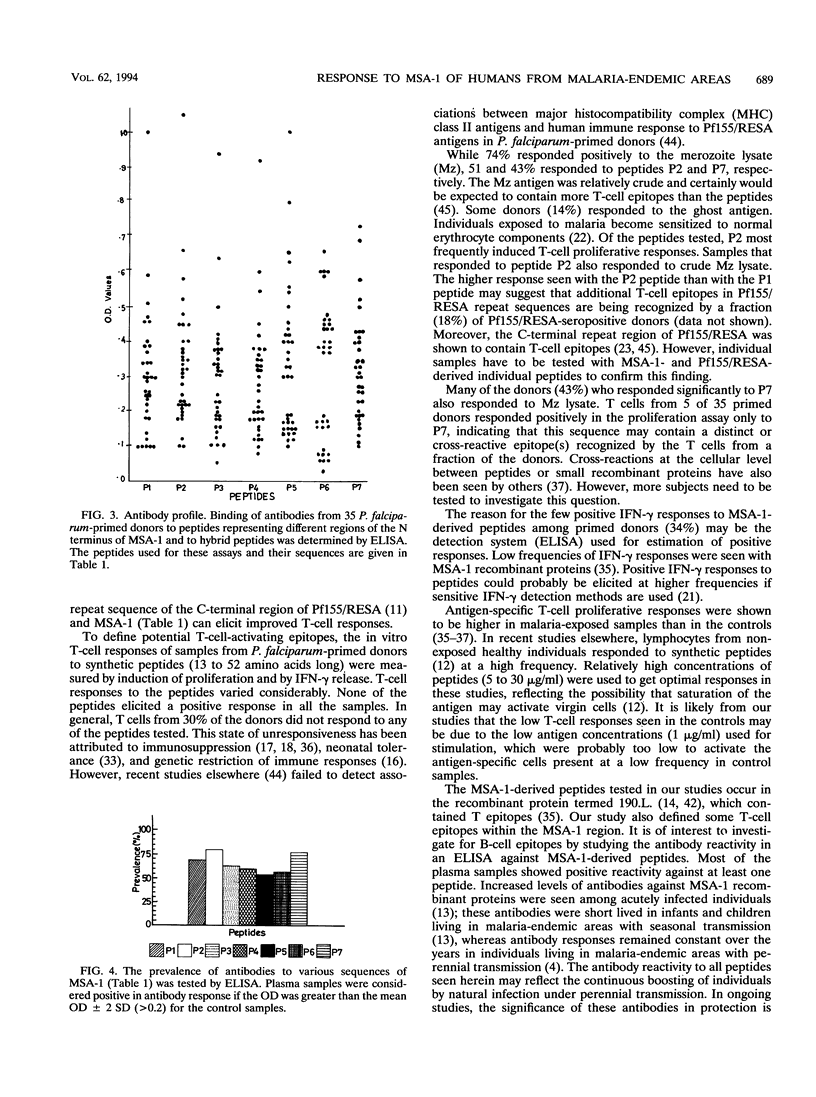
Conserved and variant regions of two blood stage vaccine candidate antigens of Plasmodium falciparum, merozoite surface antigen (MSA-1) and ring-infected erythrocyte surface antigen (Pf155/RESA), have been shown to be immunogenic. However, the relative immunogenicity of these immunogens in different populations has not been studied. The conserved N-terminal region of MSA-1 was investigated for its immunogenicity by studying cellular (T cell) and humoral (B cell) immune responses in P. falciparum-primed individuals, living in malaria-hyperendemic areas (Orissa State, India), where malaria presents an alarming situation. MSA-1-derived synthetic peptides contained sequences that activated T cells to proliferate and release gamma interferon in vitro. There was considerable variation in the responses to different peptides. However, the highest responses (51% [18 of 35] by proliferation and 34% [12 of 35] by gamma interferon release) were obtained with a synthetic hybrid peptide containing sequences from conserved N- and C-terminal repeat regions of MSA-1 and Pf155/RESA, respectively. Antibody reactivities in an enzyme immunoassay of plasma samples from these donors to different peptides used for T-cell activation were heterogeneous. In general, there was poor correlation between DNA synthesis and either gamma interferon release or antibody responses in individual donors, underlining the importance of examining several parameters of T-cell activation to assess the total T-cell responsiveness of a study population to a given antigen. However, the results from our studies suggest that synthetic constructs containing sequences from the N- and C-terminal regions of MSA-1 and Pf155/RESA representing different erythrocytic stages of the P. falciparum parasite are more immunogenic in humans living in malaria-hyperendemic areas of India who have been primed by natural infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986 Nov;8(6):529–539. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Andersson G., Ekre H. P., Alm G., Perlmann P. Monoclonal antibody two-site ELISA for human IFN-gamma. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989 Dec 20;125(1-2):89–96. doi: 10.1016/0022-1759(89)90081-1. [DOI] [PubMed] [Google Scholar]
- Berzins K., Perlmann H., Wåhlin B., Ekre H. P., Högh B., Petersen E., Wellde B., Schoenbechler M., Williams J., Chulay J. Passive immunization of Aotus monkeys with human antibodies to the Plasmodium falciparum antigen Pf155/RESA. Infect Immun. 1991 Apr;59(4):1500–1506. doi: 10.1128/iai.59.4.1500-1506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman A., Perlmann H., Petersen E., Hogh B., Lebbad M., Warsame M., Hanson A. P., Perlmann P. Consecutive determinations of seroreactivities to Pf 155/RESA antigen and to its different repetitive sequences in adult men from a holoendemic area of Liberia. Parasite Immunol. 1990 Mar;12(2):115–123. doi: 10.1111/j.1365-3024.1990.tb00941.x. [DOI] [PubMed] [Google Scholar]
- Blackman M. J., Heidrich H. G., Donachie S., McBride J. S., Holder A. A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990 Jul 1;172(1):379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. E., Webster H. K., Lyon J. A., Thomas A. W., Permpanich B., Gross M. Characterization of naturally acquired antibody responses to a recombinant fragment from the N-terminus of Plasmodium falciparum glycoprotein 195. Am J Trop Med Hyg. 1991 Nov;45(5):567–573. doi: 10.4269/ajtmh.1991.45.567. [DOI] [PubMed] [Google Scholar]
- Cavacini L. A., Parke L. A., Weidanz W. P. Resolution of acute malarial infections by T cell-dependent non-antibody-mediated mechanisms of immunity. Infect Immun. 1990 Sep;58(9):2946–2950. doi: 10.1128/iai.58.9.2946-2950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Crisanti A., Müller H. M., Hilbich C., Sinigaglia F., Matile H., McKay M., Scaife J., Beyreuther K., Bujard H. Epitopes recognized by human T cells map within the conserved part of the GP190 of P. falciparum. Science. 1988 Jun 3;240(4857):1324–1326. doi: 10.1126/science.2453924. [DOI] [PubMed] [Google Scholar]
- De Souza J. B., Playfair J. H. Immunization of mice against blood-stage Plasmodium yoelii malaria with isoelectrically focused antigens and correlation of immunity with T-cell priming in vivo. Infect Immun. 1988 Jan;56(1):88–91. doi: 10.1128/iai.56.1.88-91.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J. M., Coppel R. L., Corcoran L. M., Foote S. J., Brown G. V., Anders R. F., Kemp D. J. Structure of the RESA gene of Plasmodium falciparum. Nucleic Acids Res. 1986 Nov 11;14(21):8265–8277. doi: 10.1093/nar/14.21.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern J., Good M. F. Promiscuous malaria peptide epitope stimulates CD45Ra T cells from peripheral blood of nonexposed donors. J Immunol. 1992 Feb 1;148(3):907–913. [PubMed] [Google Scholar]
- Früh K., Doumbo O., Müller H. M., Koita O., McBride J., Crisanti A., Touré Y., Bujard H. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect Immun. 1991 Apr;59(4):1319–1324. doi: 10.1128/iai.59.4.1319-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz R., Certa U., Takacs B., Matile H., Döbeli H., Pink R., Mackay M., Bone N., Scaife J. G. Major surface antigen p190 of Plasmodium falciparum: detection of common epitopes present in a variety of plasmodia isolates. EMBO J. 1988 Jan;7(1):225–230. doi: 10.1002/j.1460-2075.1988.tb02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Miller L. H. The T cell response to the malaria circumsporozoite protein: an immunological approach to vaccine development. Annu Rev Immunol. 1988;6:663–688. doi: 10.1146/annurev.iy.06.040188.003311. [DOI] [PubMed] [Google Scholar]
- Goonewardene R., Carter R., Gamage C. P., Del Giudice G., David P. H., Howie S., Mendis K. N. Human T cell proliferative responses to Plasmodium vivax antigens: evidence of immunosuppression following prolonged exposure to endemic malaria. Eur J Immunol. 1990 Jun;20(6):1387–1391. doi: 10.1002/eji.1830200626. [DOI] [PubMed] [Google Scholar]
- Ho M., Webster H. K., Looareesuwan S., Supanaranond W., Phillips R. E., Chanthavanich P., Warrell D. A. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J Infect Dis. 1986 Apr;153(4):763–771. doi: 10.1093/infdis/153.4.763. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Sandhu J. S., Hillman Y., Davey L. S., Nicholls S. C., Cooper H., Lockyer M. J. Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology. 1987 Apr;94(Pt 2):199–208. doi: 10.1017/s0031182000053889. [DOI] [PubMed] [Google Scholar]
- Kabilan L., Troye-Blomberg M., Andersson G., Riley E. M., Ekre H. P., Whittle H. C., Perlmann P. Number of cells from Plasmodium falciparum-immune donors that produce gamma interferon in vitro in response to Pf155/RESA, a malaria vaccine candidate antigen. Infect Immun. 1990 Sep;58(9):2989–2994. doi: 10.1128/iai.58.9.2989-2994.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabilan L., Troye-Blomberg M., Patarroyo M. E., Björkman A., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria: IV. T cell dependent production of immunoglobulin and anti-P. falciparum antibodies in vitro. Clin Exp Immunol. 1987 May;68(2):288–297. [PMC free article] [PubMed] [Google Scholar]
- Kabilan L., Troye-Blomberg M., Perlmann H., Andersson G., Högh B., Petersen E., Björkman A., Perlmann P. T-cell epitopes in Pf155/RESA, a major candidate for a Plasmodium falciparum malaria vaccine. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5659–5663. doi: 10.1073/pnas.85.15.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Arora R., Kaur P., Chauhan V. S., Sharma P. "Universal" T helper cell determinants enhance immunogenicity of a Plasmodium falciparum merozoite surface antigen peptide. J Immunol. 1992 Mar 1;148(5):1499–1505. [PubMed] [Google Scholar]
- Langhorne J., Simon-Haarhaus B. Differential T cell responses to Plasmodium chabaudi chabaudi in peripheral blood and spleens of C57BL/6 mice during infection. J Immunol. 1991 Apr 15;146(8):2771–2775. [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Müller H. M., Früh K., von Brunn A., Esposito F., Lombardi S., Crisanti A., Bujard H. Development of the human immune response against the major surface protein (gp190) of Plasmodium falciparum. Infect Immun. 1989 Dec;57(12):3765–3769. doi: 10.1128/iai.57.12.3765-3769.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarroyo M. E., Amador R., Clavijo P., Moreno A., Guzman F., Romero P., Tascon R., Franco A., Murillo L. A., Ponton G. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988 Mar 10;332(6160):158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. E., Romero P., Torres M. L., Clavijo P., Moreno A., Martínez A., Rodríguez R., Guzman F., Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987 Aug 13;328(6131):629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Berzins K., Wåhlin B., Troye-Blomberg M., Hagstedt M., Andersson I., Högh B., Petersen E., Björkman A. Dissection of the human antibody response to the malaria antigen Pf155/RESA into epitope specific components. Immunol Rev. 1989 Dec;112:115–132. doi: 10.1111/j.1600-065x.1989.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Pape G. R., Halldén G. Purification, fractionation and assay of antibody-dependent lymphocytic effector cells (K cells) in human blood. Scand J Immunol. 1976 Jun;Suppl 5:57–68. doi: 10.1111/j.1365-3083.1976.tb03856.x. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Allen S. J., Wheeler J. G., Blackman M. J., Bennett S., Takacs B., Schönfeld H. J., Holder A. A., Greenwood B. M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992 May;14(3):321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Jepsen S., Andersson G., Otoo L. N., Greenwood B. M. Cell-mediated immune responses to Plasmodium falciparum antigens in adult Gambians. Clin Exp Immunol. 1988 Mar;71(3):377–382. [PMC free article] [PubMed] [Google Scholar]
- Rzepczyk C. M., Ramasamy R., Mutch D. A., Ho P. C., Battistutta D., Anderson K. L., Parkinson D., Doran T. J., Honeyman M. Analysis of human T cell response to two Plasmodium falciparum merozoite surface antigens. Eur J Immunol. 1989 Oct;19(10):1797–1802. doi: 10.1002/eji.1830191006. [DOI] [PubMed] [Google Scholar]
- Sabchareon A., Burnouf T., Ouattara D., Attanath P., Bouharoun-Tayoun H., Chantavanich P., Foucault C., Chongsuphajaisiddhi T., Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991 Sep;45(3):297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- Sharma V. P. Community-based malaria control in India. Parasitol Today. 1987 Jul;3(7):222–226. doi: 10.1016/0169-4758(87)90066-4. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia F., Takacs B., Jacot H., Matile H., Pink J. R., Crisanti A., Bujard H. Nonpolymorphic regions of p190, a protein of the Plasmodium falciparum erythrocytic stage, contain both T and B cell epitopes. J Immunol. 1988 May 15;140(10):3568–3572. [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Andersson G., Stoczkowska M., Shabo R., Romero P., Patarroyo M. E., Wigzell H., Perlmann P. Production of IL 2 and IFN-gamma by T cells from malaria patients in response to Plasmodium falciparum or erythrocyte antigens in vitro. J Immunol. 1985 Nov;135(5):3498–3504. [PubMed] [Google Scholar]
- Troye-Blomberg M., Olerup O., Larsson A., Sjöberg K., Perlmann H., Riley E., Lepers J. P., Perlmann P. Failure to detect MHC class II associations of the human immune response induced by repeated malaria infections to the Plasmodium falciparum antigen Pf155/RESA. Int Immunol. 1991 Oct;3(10):1043–1051. doi: 10.1093/intimm/3.10.1043. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Riley E. M., Perlmann H., Andersson G., Larsson A., Snow R. W., Allen S. J., Houghten R. A., Olerup O., Greenwood B. M. T and B cell responses of Plasmodium falciparum malaria-immune individuals to synthetic peptides corresponding to sequences in different regions of the P. falciparum antigen Pf155/RESA. J Immunol. 1989 Nov 1;143(9):3043–3048. [PubMed] [Google Scholar]
- Wahlgren M., Bejarano M. T., Troye-Blomberg M., Perlmann P., Riley E., Greenwood B. M., Patarroyo M. E., Gonzales C. I., Martinez A. Epitopes of the Plasmodium falciparum clustered-asparagine-rich protein (CARP) recognized by human T-cells and antibodies. Parasite Immunol. 1991 Nov;13(6):681–694. doi: 10.1111/j.1365-3024.1991.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Yadav R. S., Ghosh S. K., Chand S. K., Kumar A. Prevalence of malaria and economic loss in two major iron ore mines in Sundargarh district, Orissa. Indian J Malariol. 1991 Jun;28(2):105–113. [PubMed] [Google Scholar]
- Yadav R. S., Sharma V. P., Ghosh S. K., Kumar A. Quartan malaria--an investigation on the incidence of Plasmodium malariae in Bisra PHC, District Sundargarh, Orissa. Indian J Malariol. 1990 Jun;27(2):85–94. [PubMed] [Google Scholar]
- del Portillo H. A., Longacre S., Khouri E., David P. H. Primary structure of the merozoite surface antigen 1 of Plasmodium vivax reveals sequences conserved between different Plasmodium species. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4030–4034. doi: 10.1073/pnas.88.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]



