Abstract
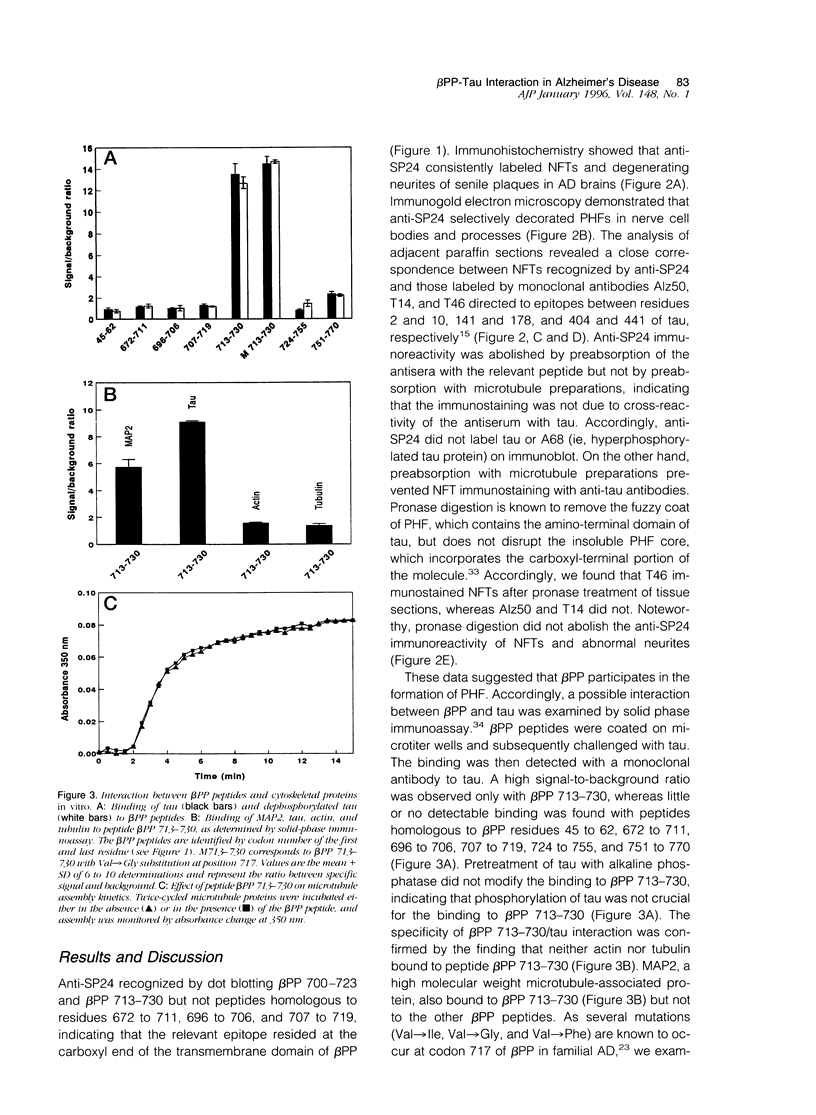
Extracellular deposition of amyloid fibrils and intraneuronal accumulation of paired helical filaments (PHFs) are the neuropathological hallmarks of Alzheimer's disease. The major constituent of amyloid fibrils is a 39- to 43-residue peptide (termed A beta), which is derived from a 695- to 770-amino-acid precursor protein (termed beta PP). The main component of PHFs identified so far is the microtubule-associated protein tau. Yet, there is no direct evidence of interconnection between these two pathological states. We report here that antibodies to an epitope located between residues 713 and 723 of beta PP770 (ie, the transmembrane region of beta PP distal to A beta) consistently labeled PHFs in the brain of Alzheimer patients. Solid phase immunoassay showed that a peptide homologous to residues 713 to 730 of beta PP770 bound tau proteins. This beta PP peptide spontaneously formed fibrils in vitro and, in the presence of tau, generated dense fibrillary assemblies containing both molecules. These data suggest that beta PP or beta PP fragments containing the tau binding site are involved in the pathogenesis of PHFs in Alzheimer's disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bondareff W., Wischik C. M., Novak M., Amos W. B., Klug A., Roth M. Molecular analysis of neurofibrillary degeneration in Alzheimer's disease. An immunohistochemical study. Am J Pathol. 1990 Sep;137(3):711–723. [PMC free article] [PubMed] [Google Scholar]
- Bramblett G. T., Goedert M., Jakes R., Merrick S. E., Trojanowski J. Q., Lee V. M. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993 Jun;10(6):1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Lorenzo A., Yeh J., Yankner B. A. beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995 Apr;14(4):879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- Buée-Scherrer V., Buée L., Hof P. R., Leveugle B., Gilles C., Loerzel A. J., Perl D. P., Delacourte A. Neurofibrillary degeneration in amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam. Immunochemical characterization of tau proteins. Am J Pathol. 1995 Apr;146(4):924–932. [PMC free article] [PubMed] [Google Scholar]
- Caputo C. B., Fraser P. E., Sobel I. E., Kirschner D. A. Amyloid-like properties of a synthetic peptide corresponding to the carboxy terminus of beta-amyloid protein precursor. Arch Biochem Biophys. 1992 Jan;292(1):199–205. doi: 10.1016/0003-9861(92)90068-8. [DOI] [PubMed] [Google Scholar]
- Caputo C. B., Sygowski L. A., Scott C. W., Sobel I. R. Role of tau in the polymerization of peptides from beta-amyloid precursor protein. Brain Res. 1992 Dec 4;597(2):227–232. doi: 10.1016/0006-8993(92)91478-w. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Olesen O. F., Smith M. J., Jakes R., Goedert M. Assembly of Alzheimer-like filaments from full-length tau protein. FEBS Lett. 1994 Jan 10;337(2):135–138. doi: 10.1016/0014-5793(94)80260-2. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goedert M., Wischik C. M., Crowther R. A., Walker J. E., Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Hof P. R., Bouras C., Buée L., Delacourte A., Perl D. P., Morrison J. H. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer's disease cases. Acta Neuropathol. 1992;85(1):23–30. doi: 10.1007/BF00304630. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron. 1994 Jul;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Kondo J., Honda T., Mori H., Hamada Y., Miura R., Ogawara M., Ihara Y. The carboxyl third of tau is tightly bound to paired helical filaments. Neuron. 1988 Nov;1(9):827–834. doi: 10.1016/0896-6273(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Structural stability of paired helical filaments requires microtubule-binding domains of tau: a model for self-association. Neuron. 1991 May;6(5):717–728. doi: 10.1016/0896-6273(91)90169-z. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migheli A., Autilio-Gambetti L., Gambetti P., Mocellini C., Vigliani M. C., Schiffer D. Ubiquitinated filamentous inclusions in spinal cord of patients with motor neuron disease. Neurosci Lett. 1990 Jun 22;114(1):5–10. doi: 10.1016/0304-3940(90)90419-a. [DOI] [PubMed] [Google Scholar]
- Mullan M., Crawford F. Genetic and molecular advances in Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):398–403. doi: 10.1016/0166-2236(93)90007-9. [DOI] [PubMed] [Google Scholar]
- Pedrotti B., Colombo R., Islam K. Interactions of microtubule-associated protein MAP2 with unpolymerized and polymerized tubulin and actin using a 96-well microtiter plate solid-phase immunoassay. Biochemistry. 1994 Jul 26;33(29):8798–8806. doi: 10.1021/bi00195a023. [DOI] [PubMed] [Google Scholar]
- Pedrotti B., Colombo R., Islam K. Microtubule associated protein MAP1A is an actin-binding and crosslinking protein. Cell Motil Cytoskeleton. 1994;29(2):110–116. doi: 10.1002/cm.970290203. [DOI] [PubMed] [Google Scholar]
- Pedrotti B., Soffientini A., Islam K. Sulphonate buffers affect the recovery of microtubule-associated proteins MAP1 and MAP2: evidence that MAP1A promotes microtubule assembly. Cell Motil Cytoskeleton. 1993;25(3):234–242. doi: 10.1002/cm.970250304. [DOI] [PubMed] [Google Scholar]
- Perry G., Cras P., Siedlak S. L., Tabaton M., Kawai M. Beta protein immunoreactivity is found in the majority of neurofibrillary tangles of Alzheimer's disease. Am J Pathol. 1992 Feb;140(2):283–290. [PMC free article] [PubMed] [Google Scholar]
- Perry G., Richey P. L., Siedlak S. L., Smith M. A., Mulvihill P., DeWitt D. A., Barnett J., Greenberg B. D., Kalaria R. N. Immunocytochemical evidence that the beta-protein precursor is an integral component of neurofibrillary tangles of Alzheimer's disease. Am J Pathol. 1993 Dec;143(6):1586–1593. [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Prelli F., Castaño E., Glenner G. G., Frangione B. Differences between vascular and plaque core amyloid in Alzheimer's disease. J Neurochem. 1988 Aug;51(2):648–651. doi: 10.1111/j.1471-4159.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Shin R. W., Bramblett G. T., Lee V. M., Trojanowski J. Q. Alzheimer disease A68 proteins injected into rat brain induce codeposits of beta-amyloid, ubiquitin, and alpha 1-antichymotrypsin. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6825–6828. doi: 10.1073/pnas.90.14.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R. W., Lee V. M., Trojanowski J. Q. Aluminum modifies the properties of Alzheimer's disease PHF tau proteins in vivo and in vitro. J Neurosci. 1994 Nov;14(11 Pt 2):7221–7233. doi: 10.1523/JNEUROSCI.14-11-07221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Siedlak S. L., Richey P. L., Mulvihill P., Ghiso J., Frangione B., Tagliavini F., Giaccone G., Bugiani O., Praprotnik D. Tau protein directly interacts with the amyloid beta-protein precursor: implications for Alzheimer's disease. Nat Med. 1995 Apr;1(4):365–369. doi: 10.1038/nm0495-365. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Giaccone G., Prelli F., Verga L., Porro M., Trojanowski J. Q., Farlow M. R., Frangione B., Ghetti B., Bugiani O. A68 is a component of paired helical filaments of Gerstmann-Sträussler-Scheinker disease, Indiana kindred. Brain Res. 1993 Jul 9;616(1-2):325–329. doi: 10.1016/0006-8993(93)90226-d. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Prelli F., Verga L., Giaccone G., Sarma R., Gorevic P., Ghetti B., Passerini F., Ghibaudi E., Forloni G. Synthetic peptides homologous to prion protein residues 106-147 form amyloid-like fibrils in vitro. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9678–9682. doi: 10.1073/pnas.90.20.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Wille H., Drewes G., Biernat J., Mandelkow E. M., Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol. 1992 Aug;118(3):573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]




