Abstract
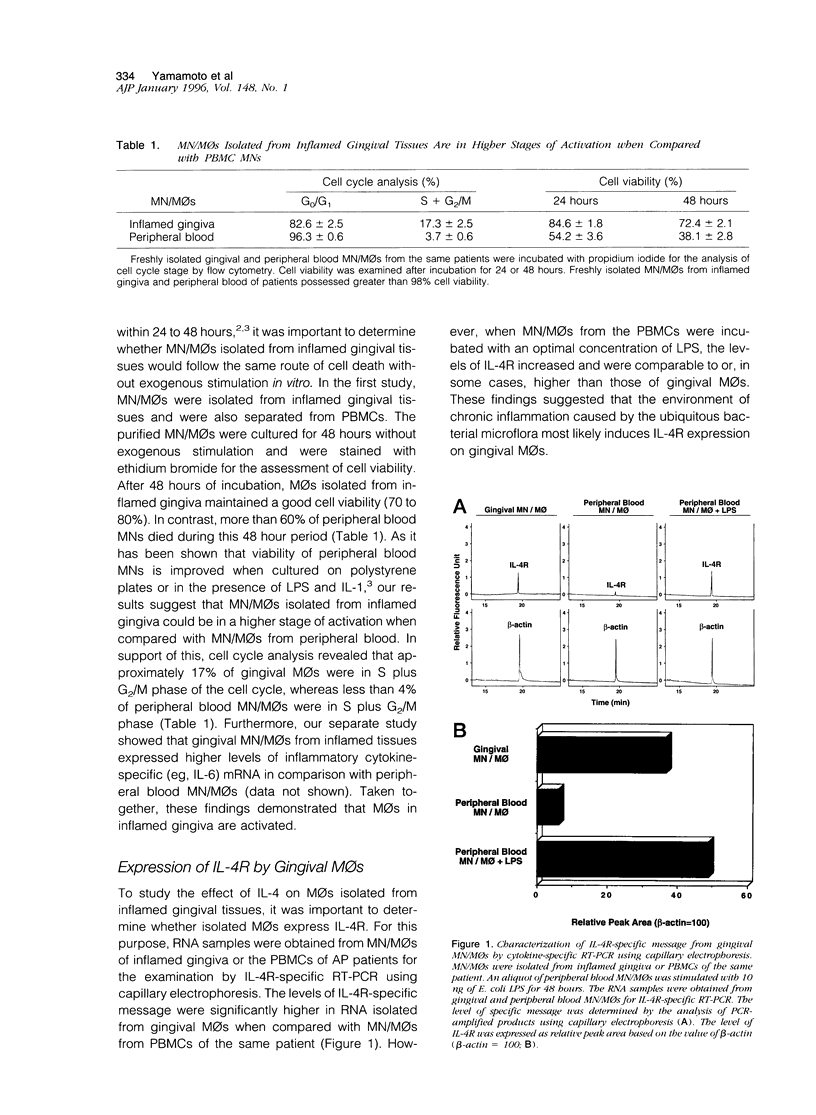
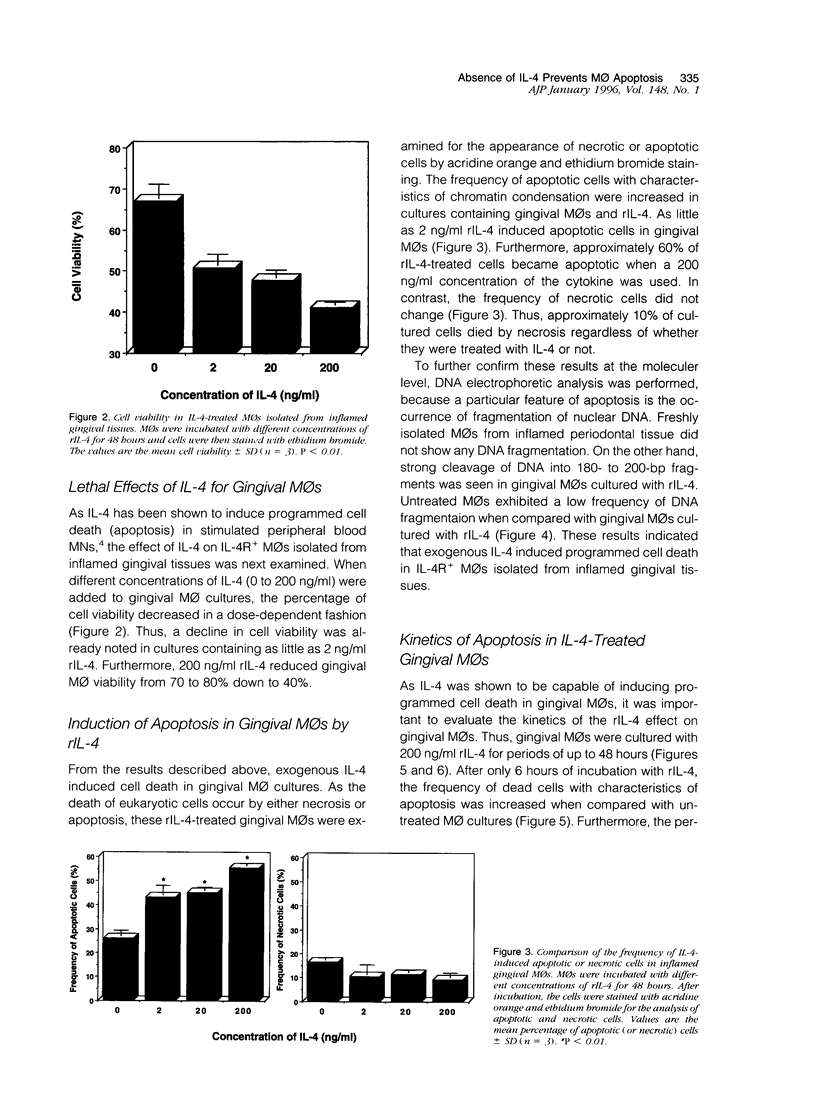
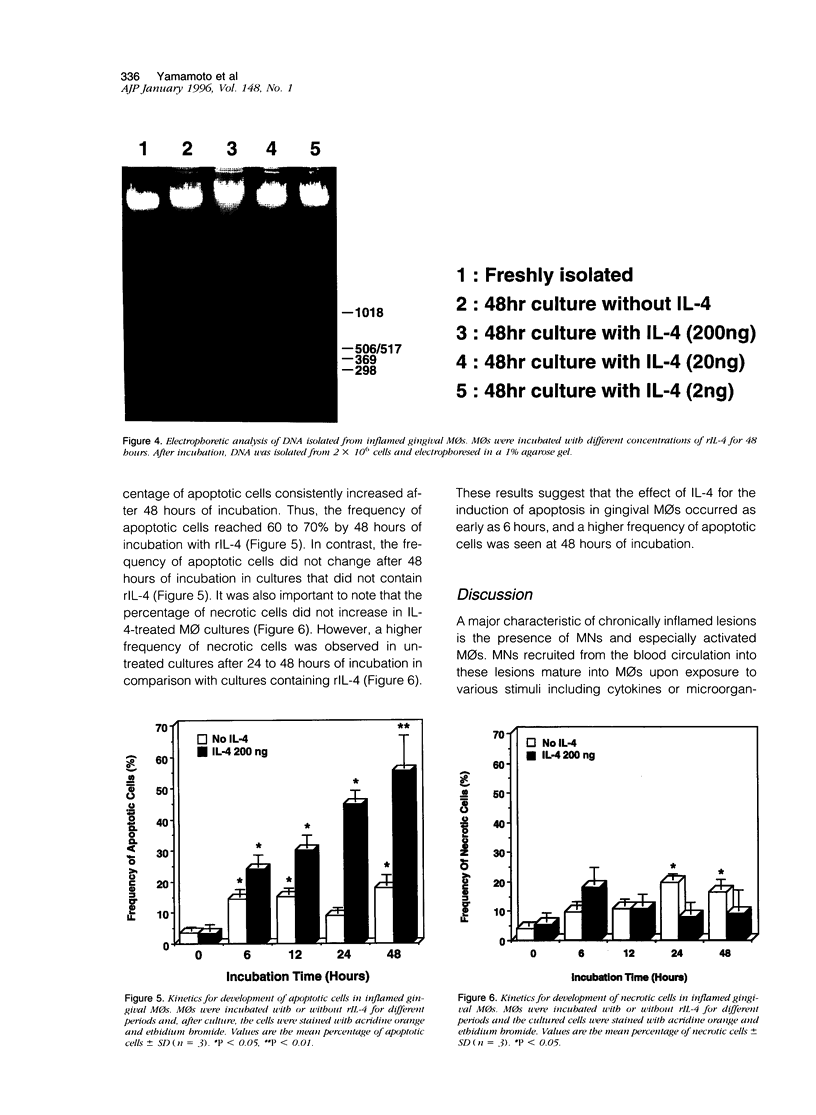
Inflamed gingival tissues are enriched in macrophages (MOs) and CD4-positive T cells; however, T helper-type cytokines such as interleukin (IL)-2 and IL-4 are absent. Therefore, we investigated whether a relationship exists between IL-4 receptor (IL-4R) expression and MO persistence in the absence of exogenous IL-4. Gingival MOs, when compared with monocyte(MN)/MOs from peripheral blood mononuclear cells, expressed high levels of IL-4R mRNA. Furthermore, in vitro cultures of gingival MOs remained viable whereas identically treated peripheral blood MN/MOs rapidly lost viability. However, when gingival MOs were incubated with recombinant IL-4 (rIL-4), the cell viability was dramatically reduced. When the frequency of apoptotic cells was assessed in rIL-4-treated gingival MO cultures, higher numbers of apoptotic cells were noted in rIL-4-treated versus control cultures. Furthermore, rIL-4-treated MOs from inflamed gingiva showed DNA fragmentation as assessed by electrophoresis. These findings clearly show that addition of exogenous rIL-4 to gingival MO cultures leads to cell death by apoptosis. This finding would suggest that topical application of rIL-4 may inhibit the persistence of MOs in adult periodontitis, which could then lead to decreased inflammation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Allen J. B., Wong H. L., Costa G. L., Bienkowski M. J., Wahl S. M. Suppression of monocyte function and differential regulation of IL-1 and IL-1ra by IL-4 contribute to resolution of experimental arthritis. J Immunol. 1993 Oct 15;151(8):4344–4351. [PubMed] [Google Scholar]
- Donnelly R. P., Fenton M. J., Finbloom D. S., Gerrard T. L. Differential regulation of IL-1 production in human monocytes by IFN-gamma and IL-4. J Immunol. 1990 Jul 15;145(2):569–575. [PubMed] [Google Scholar]
- Essner R., Rhoades K., McBride W. H., Morton D. L., Economou J. S. IL-4 down-regulates IL-1 and TNF gene expression in human monocytes. J Immunol. 1989 Jun 1;142(11):3857–3861. [PubMed] [Google Scholar]
- Fischer D. G., Golightly M. G., Koren H. S. Potentiation of the cytolytic activity of peripheral blood monocytes by lymphokines and interferon. J Immunol. 1983 Mar;130(3):1220–1225. [PubMed] [Google Scholar]
- Fujihashi K., Beagley K. W., Kono Y., Aicher W. K., Yamamoto M., DiFabio S., Xu-Amano J., McGhee J. R., Kiyono H. Gingival mononuclear cells from chronic inflammatory periodontal tissues produce interleukin (IL)-5 and IL-6 but not IL-2 and IL-4. Am J Pathol. 1993 Apr;142(4):1239–1250. [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jully J. M., Béné M. C., Martin G., Faure G. Immunohistological identification of cell subsets in human gingiva after local treatment for gingivitis or periodontitis. J Clin Periodontol. 1986 Mar;13(3):223–227. doi: 10.1111/j.1600-051x.1986.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Kelley J. L., Rozek M. M., Suenram C. A., Schwartz C. J. Activation of human blood monocytes by adherence to tissue culture plastic surfaces. Exp Mol Pathol. 1987 Jun;46(3):266–278. doi: 10.1016/0014-4800(87)90049-9. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Beagley K. W., Fujihashi K., McGhee J. R., Taga T., Hirano T., Kishimoto T., Kiyono H. Cytokine regulation of localized inflammation. Induction of activated B cells and IL-6-mediated polyclonal IgG and IgA synthesis in inflamed human gingiva. J Immunol. 1991 Mar 15;146(6):1812–1821. [PubMed] [Google Scholar]
- Lee J. D., Swisher S. G., Minehart E. H., McBride W. H., Economou J. S. Interleukin-4 downregulates interleukin-6 production in human peripheral blood mononuclear cells. J Leukoc Biol. 1990 May;47(5):475–479. doi: 10.1002/jlb.47.5.475. [DOI] [PubMed] [Google Scholar]
- Lu W., Han D. S., Yuan J., Andrieu J. M. Multi-target PCR analysis by capillary electrophoresis and laser-induced fluorescence. Nature. 1994 Mar 17;368(6468):269–271. doi: 10.1038/368269a0. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Robertson B., Wahl S. M. IL-4 enhances programmed cell death (apoptosis) in stimulated human monocytes. J Immunol. 1992 Mar 15;148(6):1812–1816. [PubMed] [Google Scholar]
- Mangan D. F., Wahl S. M. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991 Nov 15;147(10):3408–3412. [PubMed] [Google Scholar]
- Mangan D. F., Welch G. R., Wahl S. M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991 Mar 1;146(5):1541–1546. [PubMed] [Google Scholar]
- Matsuki Y., Yamamoto T., Hara K. Interleukin-1 mRNA-expressing macrophages in human chronically inflamed gingival tissues. Am J Pathol. 1991 Jun;138(6):1299–1305. [PMC free article] [PubMed] [Google Scholar]
- McGhee M. L., Ogawa T., Pitts A. M., Moldoveanu Z., Mestecky J., McGhee J. R., Kiyono H. Cellular analysis of functional mononuclear cells from chronically inflamed gingival tissue. Reg Immunol. 1989 Mar-Apr;2(2):103–110. [PubMed] [Google Scholar]
- Miossec P., Briolay J., Dechanet J., Wijdenes J., Martinez-Valdez H., Banchereau J. Inhibition of the production of proinflammatory cytokines and immunoglobulins by interleukin-4 in an ex vivo model of rheumatoid synovitis. Arthritis Rheum. 1992 Aug;35(8):874–883. doi: 10.1002/art.1780350805. [DOI] [PubMed] [Google Scholar]
- Miossec P., Naviliat M., Dupuy d'Angeac A., Sany J., Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor beta in rheumatoid synovitis. Arthritis Rheum. 1990 Aug;33(8):1180–1187. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tarkowski A., McGhee M. L., Moldoveanu Z., Mestecky J., Hirsch H. Z., Koopman W. J., Hamada S., McGhee J. R., Kiyono H. Analysis of human IgG and IgA subclass antibody-secreting cells from localized chronic inflammatory tissue. J Immunol. 1989 Feb 15;142(4):1150–1158. [PubMed] [Google Scholar]
- Standiford T. J., Strieter R. M., Chensue S. W., Westwick J., Kasahara K., Kunkel S. L. IL-4 inhibits the expression of IL-8 from stimulated human monocytes. J Immunol. 1990 Sep 1;145(5):1435–1439. [PubMed] [Google Scholar]
- Sztein M. B., Steeg P. S., Johnson H. M., Oppenheim J. J. Regulation of human peripheral blood monocyte DR antigen expression in vitro by lymphokines and recombinant interferons. J Clin Invest. 1984 Feb;73(2):556–565. doi: 10.1172/JCI111243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C. Periodontal disease. N Engl J Med. 1990 Feb 8;322(6):373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Fujihashi K., Amano M., McGhee J. R., Beagley K. W., Kiyono H. Cytokine synthesis and apoptosis by intestinal intraepithelial lymphocytes: signaling of high density alpha beta T cell receptor+ and gamma delta T cell receptor+ T cells via T cell receptor-CD3 complex results in interferon-gamma and interleukin-5 production, while low density T cells undergo DNA fragmentation. Eur J Immunol. 1994 Jun;24(6):1301–1306. doi: 10.1002/eji.1830240609. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Fujihashi K., Beagley K. W., McGhee J. R., Kiyono H. Cytokine synthesis by intestinal intraepithelial lymphocytes. Both gamma/delta T cell receptor-positive and alpha/beta T cell receptor-positive T cells in the G1 phase of cell cycle produce IFN-gamma and IL-5. J Immunol. 1993 Jan 1;150(1):106–114. [PubMed] [Google Scholar]
- Yamazaki K., Nakajima T., Gemmell E., Polak B., Seymour G. J., Hara K. IL-4- and IL-6-producing cells in human periodontal disease tissue. J Oral Pathol Med. 1994 Sep;23(8):347–353. doi: 10.1111/j.1600-0714.1994.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Zappa U., Reinking-Zappa M., Graf H., Espeland M. Cell populations and episodic periodontal attachment loss in humans. J Clin Periodontol. 1991 Aug;18(7):508–515. doi: 10.1111/j.1600-051x.1991.tb00082.x. [DOI] [PubMed] [Google Scholar]
- te Velde A. A., Huijbens R. J., Heije K., de Vries J. E., Figdor C. G. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990 Oct 1;76(7):1392–1397. [PubMed] [Google Scholar]
- te Velde A. A., Klomp J. P., Yard B. A., de Vries J. E., Figdor C. G. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988 Mar 1;140(5):1548–1554. [PubMed] [Google Scholar]



