Abstract
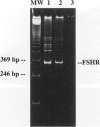
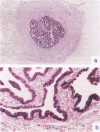
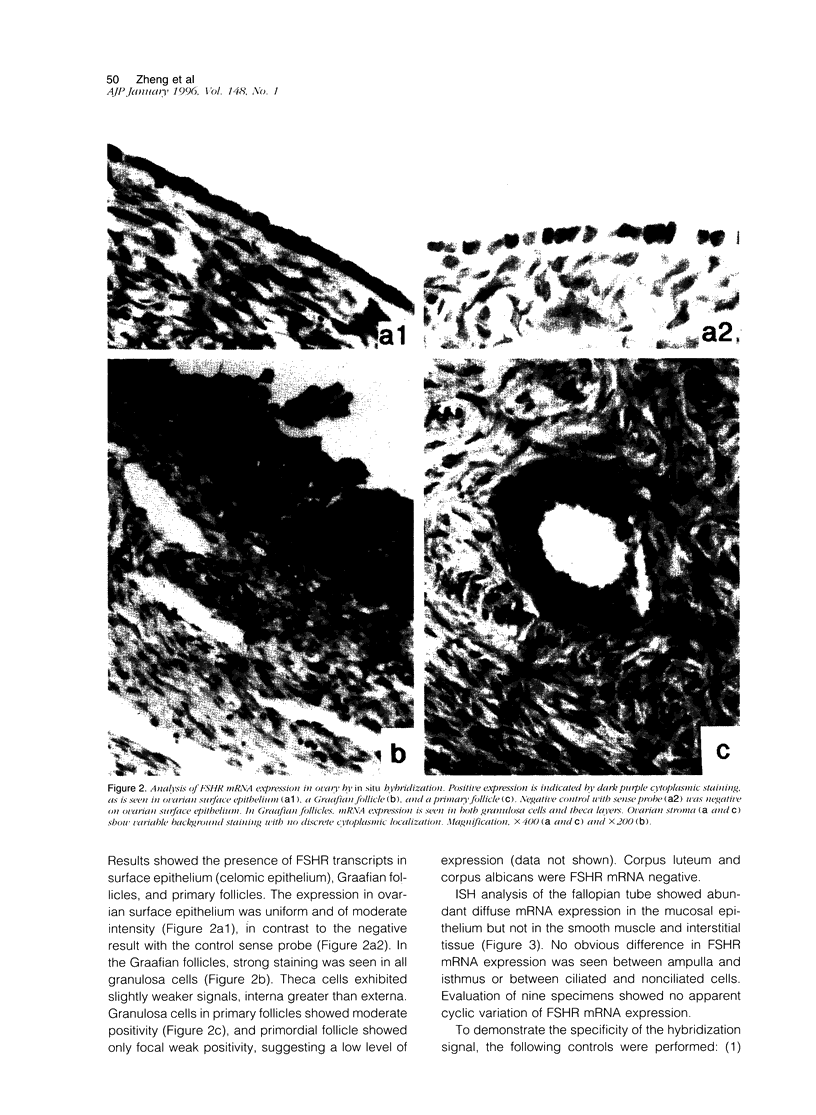
The cellular expression of pituitary gonadotropin receptors in gonadal tissues is poorly defined because of the lack of suitable reagents. In this study, we developed in situ hybridization and reverse transcription polymerase chain reaction techniques for the evaluation of follicle-stimulating hormone receptor (FSHR) expression in the ovary and fallopian tube. Using a single-strand RNA probe, we demonstrated that FSHR mRNA expression is strongest in Graafian follicles. Within these developing follicles, granulosa cells showed the greatest expression, although both theca interna and theca externa were also positive, interna greater than externa. Granulosa cells in both primary and primordial follicles were positive, with primordial follicles showing only weak focal positivity. Ovarian surface epithelium and fallopian tube epithelium, not previously recognized to express FSHR, were both strongly positive. The FSHR expression in the ovary and fallopian tube was confirmed by reverse transcription polymerase chain reaction. Our results indicated that the FSHR is expressed in a cell-specific fashion at different stages of follicular development and is also expressed in ovarian surface and fallopian tube epithelia. The presence of FSHR in ovarian surface epithelium and of gonadotropin-binding sites in ovarian neoplasms provide additional evidence supporting the derivation of epithelial ovarian tumors from the surface epithelium and should promote heightened interest in the gonadotropin theory of ovarian tumorigenesis. More importantly, this study shows the feasibility of evaluating FSHR expression by both in situ hybridization and reverse transcription polymerase chain reaction. Application of these techniques to tumor specimens will help to elucidate the role of gonadotropins and their receptors in the carcinogenesis of gynecological tumors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISKIND G. R., BERNSTEIN D. E., GOSPE S. M. The effect of exogenous gonadotrophins on the development of experimental ovarian tumors in rats. Cancer Res. 1953 Mar;13(3):216–220. [PubMed] [Google Scholar]
- Camp T. A., Rahal J. O., Mayo K. E. Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol Endocrinol. 1991 Oct;5(10):1405–1417. doi: 10.1210/mend-5-10-1405. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Kammerman S. Binding of gonadotropins to ovarian cells. Biol Reprod. 1974 Mar;10(2):179–198. doi: 10.1095/biolreprod10.2.179. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davy M., Torjesen P. A., Aakavaag A. Demonstration of an FSH receptor in a functioning granulosa cell tumour. The effect of gonadotrophin treatment on its viability following transplantation to nude mice. Acta Endocrinol (Copenh) 1977 Jul;85(3):615–623. [PubMed] [Google Scholar]
- DiZerega G. S., Richardson C. L., Davies T. F., Hodgen G. D., Catt K. J. Fluorescence localization of luteinizing hormone/human chorionic gonadotropin uptake in the primate ovary: characterizatin of the preovulatory ovary. Fertil Steril. 1980 Oct;34(4):379–385. doi: 10.1016/s0015-0282(16)45008-9. [DOI] [PubMed] [Google Scholar]
- Heckert L. L., Daley I. J., Griswold M. D. Structural organization of the follicle-stimulating hormone receptor gene. Mol Endocrinol. 1992 Jan;6(1):70–80. doi: 10.1210/mend.6.1.1738373. [DOI] [PubMed] [Google Scholar]
- Heckert L. L., Griswold M. D. Expression of follicle-stimulating hormone receptor mRNA in rat testes and Sertoli cells. Mol Endocrinol. 1991 May;5(5):670–677. doi: 10.1210/mend-5-5-670. [DOI] [PubMed] [Google Scholar]
- Hu Z. Z., Tsai-Morris C. H., Buczko E., Dufau M. L. Hormonal regulation of LH receptor mRNA and expression in the rat ovary. FEBS Lett. 1990 Nov 12;274(1-2):181–184. doi: 10.1016/0014-5793(90)81359-v. [DOI] [PubMed] [Google Scholar]
- Kammerman S., Demopoulos R. I., Raphael C., Ross J. Gonadotropic hormone binding to human ovarian tumors. Hum Pathol. 1981 Oct;12(10):886–890. doi: 10.1016/s0046-8177(81)80192-x. [DOI] [PubMed] [Google Scholar]
- Kammerman S., Demopoulos R. I., Ross J. Gonadotropin receptors in experimentally induced ovarian tumors in mice. Cancer Res. 1977 Aug;37(8 Pt 1):2578–2582. [PubMed] [Google Scholar]
- Lei Z. M., Toth P., Rao C. V., Pridham D. Novel coexpression of human chorionic gonadotropin (hCG)/human luteinizing hormone receptors and their ligand hCG in human fallopian tubes. J Clin Endocrinol Metab. 1993 Sep;77(3):863–872. doi: 10.1210/jcem.77.3.7690366. [DOI] [PubMed] [Google Scholar]
- Lindblom B., Hamberger L., Ljung B. Contractile patterns of isolated oviductal smooth muscle under different hormonal conditions. Fertil Steril. 1980 Mar;33(3):283–287. doi: 10.1016/s0015-0282(16)44595-4. [DOI] [PubMed] [Google Scholar]
- McFarland K. C., Sprengel R., Phillips H. S., Köhler M., Rosemblit N., Nikolics K., Segaloff D. L., Seeburg P. H. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science. 1989 Aug 4;245(4917):494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- Minegishi T., Nakamura K., Takakura Y., Ibuki Y., Igarashi M., Minegish T [corrected to Minegishi T. ]. Cloning and sequencing of human FSH receptor cDNA. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1125–1130. doi: 10.1016/0006-291x(91)91682-3. [DOI] [PubMed] [Google Scholar]
- Nakano R., Kitayama S., Yamoto M., Shima K., Ooshima A. Localization of gonadotropin binding sites in human ovarian neoplasms. Am J Obstet Gynecol. 1989 Oct;161(4):905–910. doi: 10.1016/0002-9378(89)90749-7. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Chen Y. T., Old L. J. A mouse mutant p53 product recognized by CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3171–3175. doi: 10.1073/pnas.91.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaniemi H., Kauppila A., Rönnberg L., Selander K., Pystynen P. LH(hCG) receptor in benign and malignant tumors of human ovary. Acta Obstet Gynecol Scand Suppl. 1981;101:83–86. doi: 10.3109/00016348109157818. [DOI] [PubMed] [Google Scholar]
- Richards J. S. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980 Jan;60(1):51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- Segaloff D. L., Sprengel R., Nikolics K., Ascoli M. Structure of the lutropin/choriogonadotropin receptor. Recent Prog Horm Res. 1990;46:261–303. doi: 10.1016/b978-0-12-571146-3.50014-6. [DOI] [PubMed] [Google Scholar]
- Simon W. E., Albrecht M., Hänsel M., Dietel M., Hölzel F. Cell lines derived from human ovarian carcinomas: growth stimulation by gonadotropic and steroid hormones. J Natl Cancer Inst. 1983 May;70(5):839–845. [PubMed] [Google Scholar]
- Sprengel R., Braun T., Nikolics K., Segaloff D. L., Seeburg P. H. The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol Endocrinol. 1990 Apr;4(4):525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- Stadel B. V. Letter: The etiology and prevention of ovarian cancer. Am J Obstet Gynecol. 1975 Dec 1;123(7):772–774. doi: 10.1016/0002-9378(75)90509-8. [DOI] [PubMed] [Google Scholar]
- Stouffer R. L., Grodin M. S., Davis J. R., Surwit E. A. Investigation of binding sites for follicle-stimulating hormone and chorionic gonadotropin in human ovarian cancers. J Clin Endocrinol Metab. 1984 Sep;59(3):441–446. doi: 10.1210/jcem-59-3-441. [DOI] [PubMed] [Google Scholar]
- Tilly J. L., Aihara T., Nishimori K., Jia X. C., Billig H., Kowalski K. I., Perlas E. A., Hsueh A. J. Expression of recombinant human follicle-stimulating hormone receptor: species-specific ligand binding, signal transduction, and identification of multiple ovarian messenger ribonucleic acid transcripts. Endocrinology. 1992 Aug;131(2):799–806. doi: 10.1210/endo.131.2.1322283. [DOI] [PubMed] [Google Scholar]
- Tonetta S. A., diZerega G. S. Intragonadal regulation of follicular maturation. Endocr Rev. 1989 May;10(2):205–229. doi: 10.1210/edrv-10-2-205. [DOI] [PubMed] [Google Scholar]
- Verhage H. G., Bareither M. L., Jaffe R. C., Akbar M. Cyclic changes in ciliation, secretion and cell height of the oviductal epithelium in women. Am J Anat. 1979 Dec;156(4):505–521. doi: 10.1002/aja.1001560405. [DOI] [PubMed] [Google Scholar]
- Wardlaw S., Lauersen N. H., Saxena B. B. The lh-hcg receptor of human ovary at various stages of the menstrual cycle. Acta Endocrinol (Copenh) 1975 Jul;79(3):568–576. doi: 10.1530/acta.0.0790568. [DOI] [PubMed] [Google Scholar]
- Wimalasena J., Dostal R., Meehan D. Gonadotropins, estradiol, and growth factors regulate epithelial ovarian cancer cell growth. Gynecol Oncol. 1992 Sep;46(3):345–350. doi: 10.1016/0090-8258(92)90230-g. [DOI] [PubMed] [Google Scholar]