Abstract
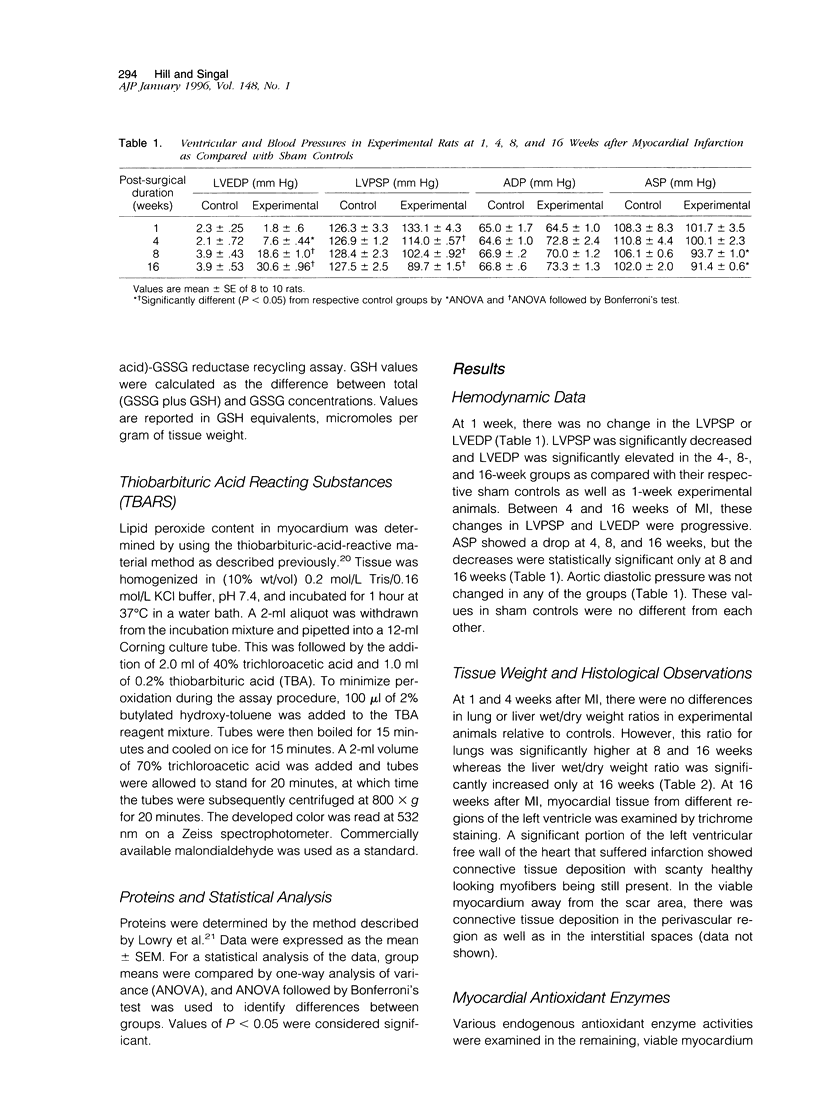
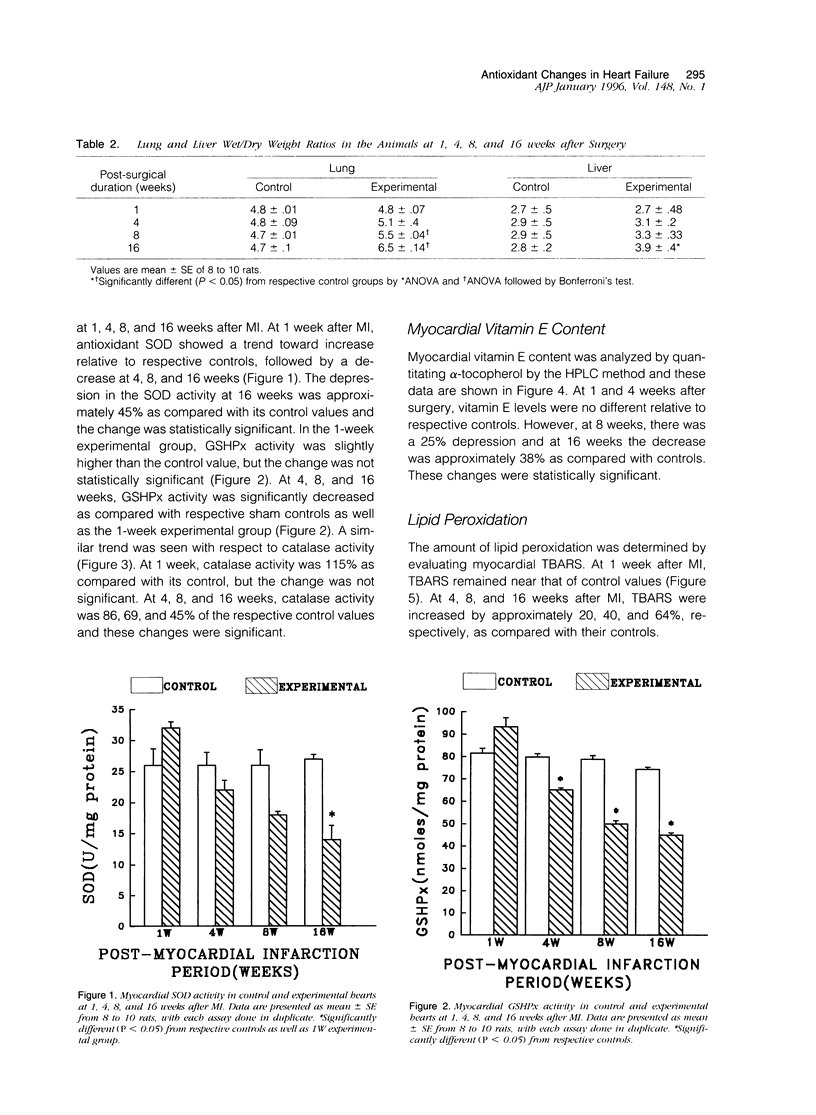
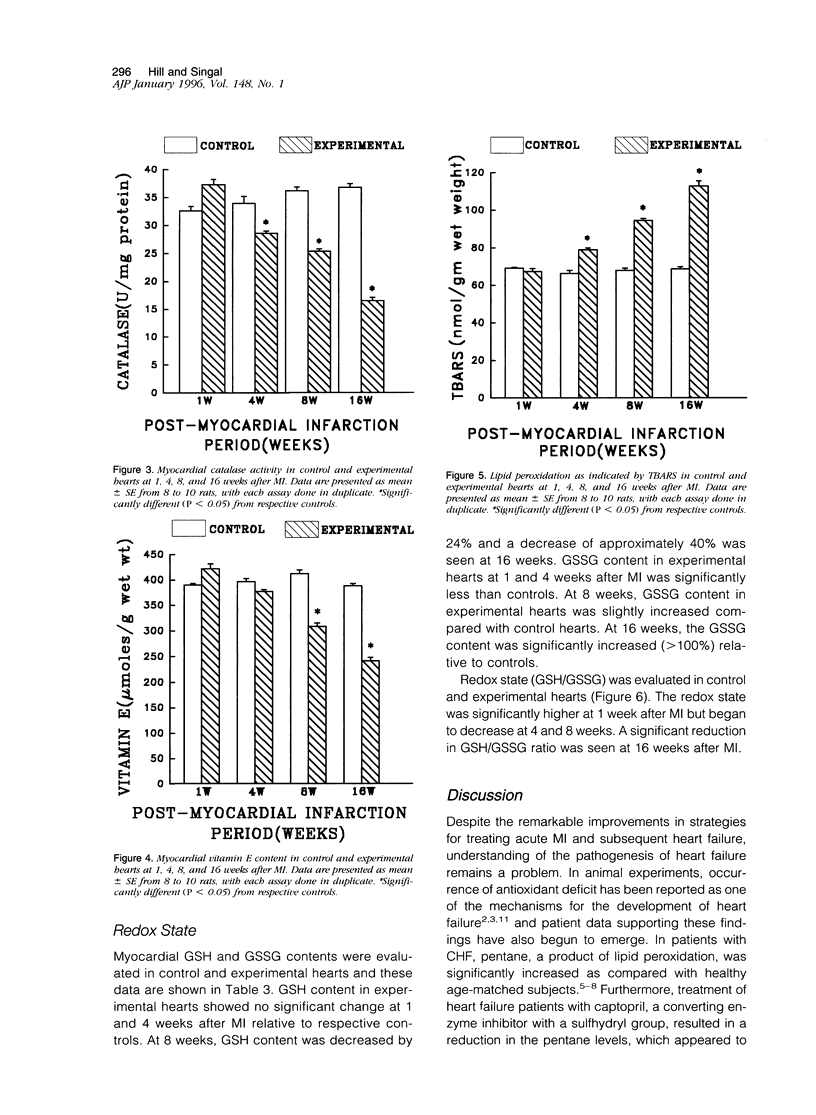
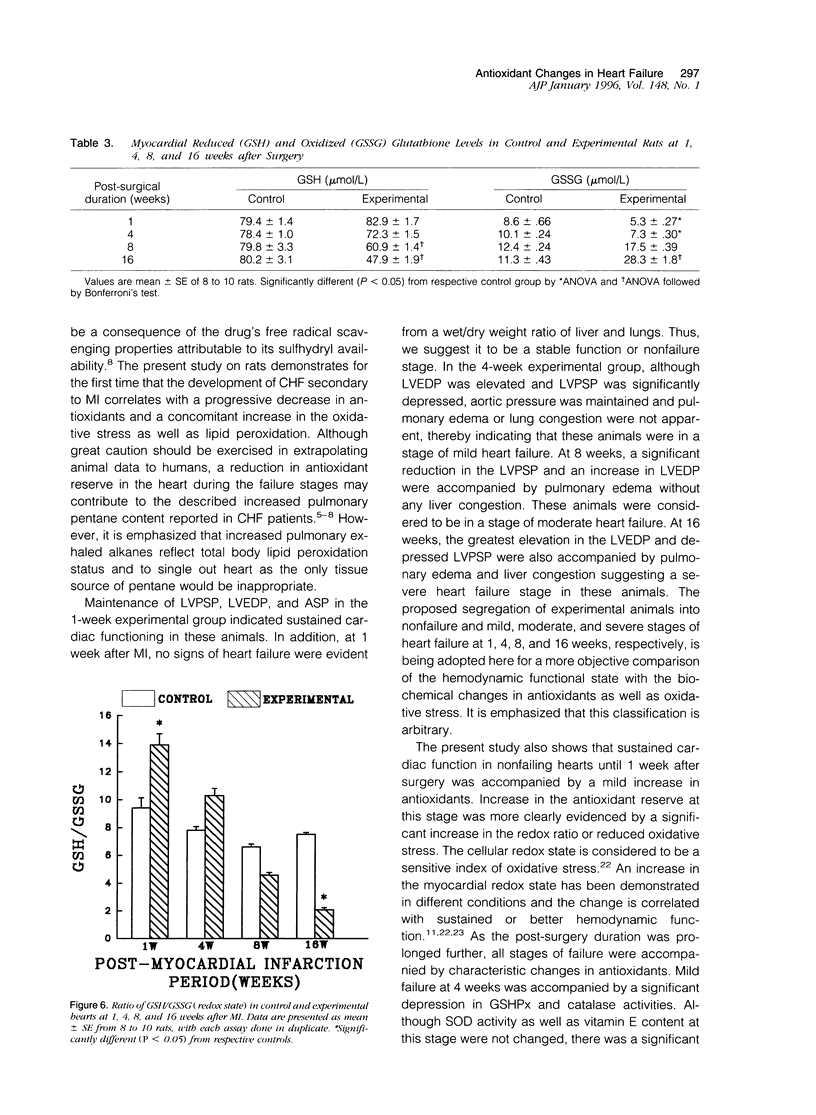
Antioxidant enzyme activities and oxidative stress were evaluated in the myocardium in relation to hemodynamic function subsequent to myocardial infarction in rats. One week after the coronary ligation, the left ventricular peak systolic pressure, left ventricular end-diastolic pressure, and aortic pressures remained near control values and there were no differences in lung and liver wet/dry weight ratios between experimental and control animals. In the 4-, 8-, and 16-week experimental animals, there was a progressive drop in left ventricular peak systolic pressure and an increase in left ventricular end-diastolic pressure. Aortic systolic pressure was depressed at 8 and 16 weeks. In myocardial infarct rats, there was a significant increase in wet/dry weight ratio of lungs at 8 weeks and at 16 weeks; this ratio was increased for lungs as well as liver. Based on the hemodynamic data as well as other observations, animals in the 1-, 4-, 8-, and 16-week groups were arbitrarily categorized into nonfailure and mild, moderate, and severe failure stages, respectively. In the nonfailure stage, there was a marginal increase in superoxide dismutase, glutathione peroxidase, and catalase activities as well as vitamin E levels. The redox state in these hearts, assessed by the reduced/oxidized glutathione ratio, was significantly increased. Superoxide dismutase activity was unchanged in mild and moderate failure stages but significantly depressed at 16 weeks. Glutathione peroxidase and catalase activities showed progressive decreases through mild, moderate, and severe failure stages. Vitamin E levels were significantly depressed at moderate and severe failure stages. There was a progressive increase in lipid peroxidation at mild, moderate, and severe stages of heart failure and the redox ratio was significantly depressed in the severe failure stage. These data suggest that heart failure subsequent to myocardial infarction may be associated with an antioxidant deficit as well as increased myocardial oxidative stress.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Grosso M. A., Terada L. S., Beehler C. J., Toth K. M., Whitman G. J., Harken A. H., Repine J. E. Erythrocytes decrease myocardial hydrogen peroxide levels and reperfusion injury. Am J Physiol. 1989 Feb;256(2 Pt 2):H584–H588. doi: 10.1152/ajpheart.1989.256.2.H584. [DOI] [PubMed] [Google Scholar]
- Ceconi C., Cargnoni A., Pasini E., Condorelli E., Curello S., Ferrari R. Evaluation of phospholipid peroxidation as malondialdehyde during myocardial ischemia and reperfusion injury. Am J Physiol. 1991 Apr;260(4 Pt 2):H1057–H1061. doi: 10.1152/ajpheart.1991.260.4.H1057. [DOI] [PubMed] [Google Scholar]
- Chow C. K., Tappel A. L. An enzymatic protective mechanism against lipid peroxidation damage to lungs of ozone-exposed rats. Lipids. 1972 Aug;7(8):518–524. doi: 10.1007/BF02533017. [DOI] [PubMed] [Google Scholar]
- Cowan D. B., Weisel R. D., Williams W. G., Mickle D. A. Identification of oxygen responsive elements in the 5'-flanking region of the human glutathione peroxidase gene. J Biol Chem. 1993 Dec 25;268(36):26904–26910. [PubMed] [Google Scholar]
- Dhaliwal H., Kirshenbaum L. A., Randhawa A. K., Singal P. K. Correlation between antioxidant changes during hypoxia and recovery on reoxygenation. Am J Physiol. 1991 Sep;261(3 Pt 2):H632–H638. doi: 10.1152/ajpheart.1991.261.3.H632. [DOI] [PubMed] [Google Scholar]
- Dixon I. M., Lee S. L., Dhalla N. S. Nitrendipine binding in congestive heart failure due to myocardial infarction. Circ Res. 1990 Mar;66(3):782–788. doi: 10.1161/01.res.66.3.782. [DOI] [PubMed] [Google Scholar]
- Fantini G. A., Yoshioka T. Use and limitations of thiobarbituric acid reaction to detect lipid peroxidation. Am J Physiol. 1992 Sep;263(3 Pt 2):H981–H983. doi: 10.1152/ajpheart.1992.263.3.H981. [DOI] [PubMed] [Google Scholar]
- Gerli G. C., Beretta L., Bianchi M., Pellegatta A., Agostoni A. Erythrocyte superoxide dismutase, catalase and glutathione peroxidase activities in beta-thalassaemia (major and minor). Scand J Haematol. 1980 Jul;25(1):87–92. doi: 10.1111/j.1600-0609.1981.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Gupta M., Singal P. K. Higher antioxidative capacity during a chronic stable heart hypertrophy. Circ Res. 1989 Feb;64(2):398–406. doi: 10.1161/01.res.64.2.398. [DOI] [PubMed] [Google Scholar]
- Gupta M., Singal P. K. Time course of structure, function, and metabolic changes due to an exogenous source of oxygen metabolites in rat heart. Can J Physiol Pharmacol. 1989 Dec;67(12):1549–1559. doi: 10.1139/y89-250. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Cartier L. J., Chen M., Holloszy J. O. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol. 1985 May;40(3):281–286. doi: 10.1093/geronj/40.3.281. [DOI] [PubMed] [Google Scholar]
- Kanter M. M., Hamlin R. L., Unverferth D. V., Davis H. W., Merola A. J. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol (1985) 1985 Oct;59(4):1298–1303. doi: 10.1152/jappl.1985.59.4.1298. [DOI] [PubMed] [Google Scholar]
- Kaul N., Siveski-Iliskovic N., Hill M., Slezak J., Singal P. K. Free radicals and the heart. J Pharmacol Toxicol Methods. 1993 Oct;30(2):55–67. doi: 10.1016/1056-8719(93)90008-3. [DOI] [PubMed] [Google Scholar]
- Kimball R. E., Reddy K., Peirce T. H., Schwartz L. W., Mustafa M. G., Cross C. E. Oxygen toxicity: augmentation of antioxidant defense mechanisms in rat lung. Am J Physiol. 1976 May;230(5):1425–1431. doi: 10.1152/ajplegacy.1976.230.5.1425. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum L. A., Singal P. K. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am J Physiol. 1993 Aug;265(2 Pt 2):H484–H493. doi: 10.1152/ajpheart.1993.265.2.H484. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Yamashita T., Kaneko M., Nishiyama T., Hayashi H., Yamazaki N. Effects of verapamil on experimental cardiomyopathy in the Bio 14.6 Syrian hamster. J Am Coll Cardiol. 1987 Nov;10(5):1128–1138. doi: 10.1016/s0735-1097(87)80356-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang J. K., Gohil K., Packer L. Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal Biochem. 1986 Aug 15;157(1):106–116. doi: 10.1016/0003-2697(86)90203-4. [DOI] [PubMed] [Google Scholar]
- Lemoyne M., Van Gossum A., Kurian R., Ostro M., Axler J., Jeejeebhoy K. N. Breath pentane analysis as an index of lipid peroxidation: a functional test of vitamin E status. Am J Clin Nutr. 1987 Aug;46(2):267–272. doi: 10.1093/ajcn/46.2.267. [DOI] [PubMed] [Google Scholar]
- McMurray J., McLay J., Chopra M., Bridges A., Belch J. J. Evidence for enhanced free radical activity in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1990 May 15;65(18):1261–1262. doi: 10.1016/0002-9149(90)90985-a. [DOI] [PubMed] [Google Scholar]
- Nohl H., Hegner D., Summer K. H. Responses of mitochondrial superoxide dismutase, catalase and glutathione peroxidase activities to aging. Mech Ageing Dev. 1979 Oct;11(3):145–151. doi: 10.1016/0047-6374(79)90050-2. [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Ratnoff O. D. Some recent advances in the study of hemostasis. Circ Res. 1974 Jul;35(1):1–14. doi: 10.1161/01.res.35.1.1. [DOI] [PubMed] [Google Scholar]
- Roberts M. J., Young I. S., Trouton T. G., Trimble E. R., Khan M. M., Webb S. W., Wilson C. M., Patterson G. C., Adgey A. A. Transient release of lipid peroxides after coronary artery balloon angioplasty. Lancet. 1990 Jul 21;336(8708):143–145. doi: 10.1016/0140-6736(90)91661-s. [DOI] [PubMed] [Google Scholar]
- SELYE H., BAJUSZ E., GRASSO S., MENDELL P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960 Oct;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- Shan X. Q., Aw T. Y., Jones D. P. Glutathione-dependent protection against oxidative injury. Pharmacol Ther. 1990;47(1):61–71. doi: 10.1016/0163-7258(90)90045-4. [DOI] [PubMed] [Google Scholar]
- Singal P. K., Dhalla A. K., Hill M., Thomas T. P. Endogenous antioxidant changes in the myocardium in response to acute and chronic stress conditions. Mol Cell Biochem. 1993 Dec 22;129(2):179–186. doi: 10.1007/BF00926366. [DOI] [PubMed] [Google Scholar]
- Singal P. K., Kapur N., Dhillon K. S., Beamish R. E., Dhalla N. S. Role of free radicals in catecholamine-induced cardiomyopathy. Can J Physiol Pharmacol. 1982 Nov;60(11):1390–1397. doi: 10.1139/y82-207. [DOI] [PubMed] [Google Scholar]
- Singal P. K., Kirshenbaum L. A. A relative deficit in antioxidant reserve may contribute in cardiac failure. Can J Cardiol. 1990 Mar;6(2):47–49. [PubMed] [Google Scholar]
- Singal P. K., Pierce G. N. Adriamycin stimulates low-affinity Ca2+ binding and lipid peroxidation but depresses myocardial function. Am J Physiol. 1986 Mar;250(3 Pt 2):H419–H425. doi: 10.1152/ajpheart.1986.250.3.H419. [DOI] [PubMed] [Google Scholar]
- Singal P. K., Tong J. G. Vitamin E deficiency accentuates adriamycin-induced cardiomyopathy and cell surface changes. Mol Cell Biochem. 1988 Dec;84(2):163–171. doi: 10.1007/BF00421051. [DOI] [PubMed] [Google Scholar]
- Siveski-Iliskovic N., Kaul N., Singal P. K. Probucol promotes endogenous antioxidants and provides protection against adriamycin-induced cardiomyopathy in rats. Circulation. 1994 Jun;89(6):2829–2835. doi: 10.1161/01.cir.89.6.2829. [DOI] [PubMed] [Google Scholar]
- Sobotka P. A., Brottman M. D., Weitz Z., Birnbaum A. J., Skosey J. L., Zarling E. J. Elevated breath pentane in heart failure reduced by free radical scavenger. Free Radic Biol Med. 1993 Jun;14(6):643–647. doi: 10.1016/0891-5849(93)90145-k. [DOI] [PubMed] [Google Scholar]
- Weitz Z. W., Birnbaum A. J., Sobotka P. A., Zarling E. J., Skosey J. L. High breath pentane concentrations during acute myocardial infarction. Lancet. 1991 Apr 20;337(8747):933–935. doi: 10.1016/0140-6736(91)91569-g. [DOI] [PubMed] [Google Scholar]


