Abstract
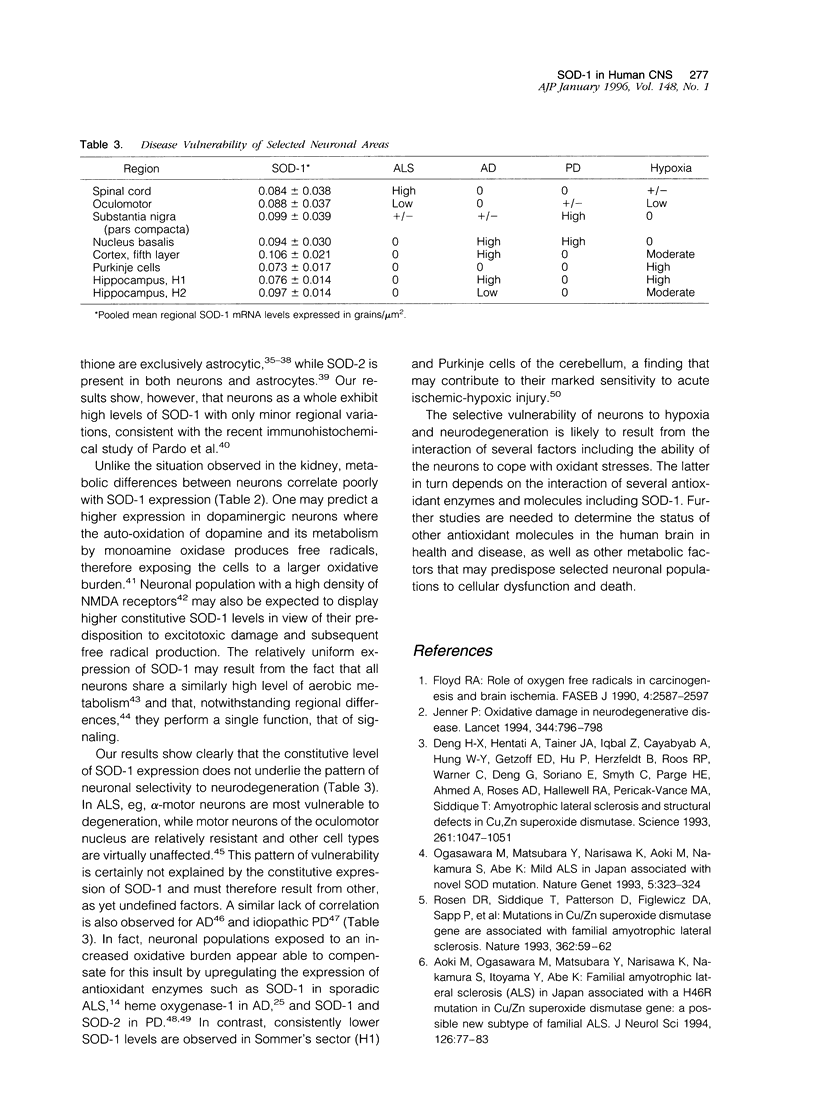
Oxidative stress has been implicated in the pathogenesis of several neurological disorders. We examined the regional distribution of copper/zinc superoxide dismutase (SOD-1), one of the key antioxidant enzymes, in the human central nervous system using in situ hybridization. Our results show that the enzyme is present at high levels of constitutive expression in alpha-motor neurons, oculomotor neurons, nucleus basalis, substantia nigra, neocortex, and the hippocampal sector resistant to hypoxia (H2). Relatively lower levels were found in Sommer's sector (H1) and Purkinje cells. We conclude that a lower constitutive level of SOD-1 expression may play a role in the selective vulnerability of certain neuronal populations to hypoxia but does not correlate with the patterns of neurodegeneration observed in amyotrophic lateral sclerosis. Parkinson's disease, and Alzheimer's disease.
Full text
PDF
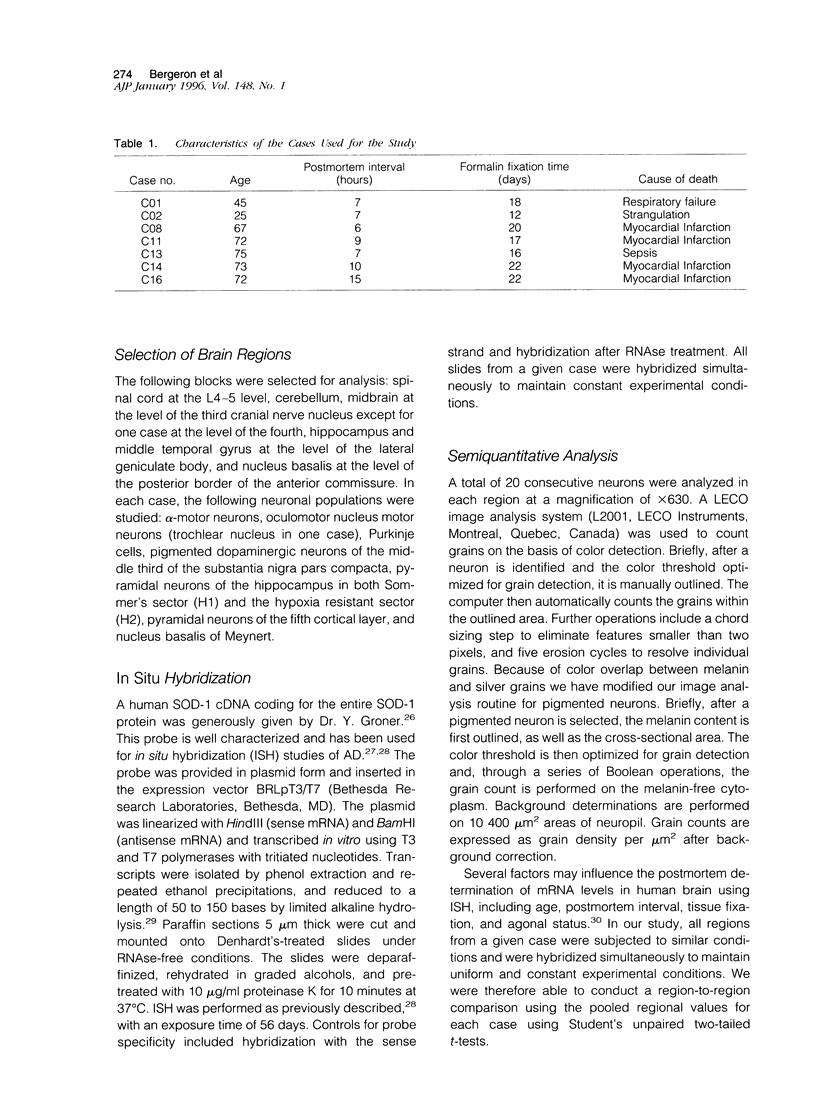


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki M., Ogasawara M., Matsubara Y., Narisawa K., Nakamura S., Itoyama Y., Abe K. Familial amyotrophic lateral sclerosis (ALS) in Japan associated with H46R mutation in Cu/Zn superoxide dismutase gene: a possible new subtype of familial ALS. J Neurol Sci. 1994 Oct;126(1):77–83. doi: 10.1016/0022-510x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Aoki M., Ogasawara M., Matsubara Y., Narisawa K., Nakamura S., Itoyama Y., Abe K. Mild ALS in Japan associated with novel SOD mutation. Nat Genet. 1993 Dec;5(4):323–324. doi: 10.1038/ng1293-323. [DOI] [PubMed] [Google Scholar]
- Barton A. J., Pearson R. C., Najlerahim A., Harrison P. J. Pre- and postmortem influences on brain RNA. J Neurochem. 1993 Jul;61(1):1–11. doi: 10.1111/j.1471-4159.1993.tb03532.x. [DOI] [PubMed] [Google Scholar]
- Behl C., Davis J. B., Lesley R., Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994 Jun 17;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Bergeron C., Muntasser S., Somerville M. J., Weyer L., Percy M. E. Copper/zinc superoxide dismutase mRNA levels are increased in sporadic amyotrophic lateral sclerosis motorneurons. Brain Res. 1994 Oct 3;659(1-2):272–276. doi: 10.1016/0006-8993(94)90892-3. [DOI] [PubMed] [Google Scholar]
- Bowling A. C., Schulz J. B., Brown R. H., Jr, Beal M. F. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993 Dec;61(6):2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Butterfield D. A., Hensley K., Harris M., Mattson M., Carney J. beta-Amyloid peptide free radical fragments initiate synaptosomal lipoperoxidation in a sequence-specific fashion: implications to Alzheimer's disease. Biochem Biophys Res Commun. 1994 Apr 29;200(2):710–715. doi: 10.1006/bbrc.1994.1508. [DOI] [PubMed] [Google Scholar]
- Ceballos I., Javoy-Agid F., Delacourte A., Defossez A., Lafon M., Hirsch E., Nicole A., Sinet P. M., Agid Y. Neuronal localization of copper-zinc superoxide dismutase protein and mRNA within the human hippocampus from control and Alzheimer's disease brains. Free Radic Res Commun. 1991;12-13 Pt 2:571–580. doi: 10.3109/10715769109145832. [DOI] [PubMed] [Google Scholar]
- Damier P., Hirsch E. C., Zhang P., Agid Y., Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience. 1993 Jan;52(1):1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Elshafey A., Lanyon W. G., Connor J. M. Identification of a new missense point mutation in exon 4 of the Cu/Zn superoxide dismutase (SOD-1) gene in a family with amyotrophic lateral sclerosis. Hum Mol Genet. 1994 Feb;3(2):363–364. doi: 10.1093/hmg/3.2.363. [DOI] [PubMed] [Google Scholar]
- Esteban J., Rosen D. R., Bowling A. C., Sapp P., McKenna-Yasek D., O'Regan J. P., Beal M. F., Horvitz H. R., Brown R. H., Jr Identification of two novel mutations and a new polymorphism in the gene for Cu/Zn superoxide dismutase in patients with amyotrophic lateral sclerosis. Hum Mol Genet. 1994 Jun;3(6):997–998. doi: 10.1093/hmg/3.6.997. [DOI] [PubMed] [Google Scholar]
- FRIEDE R. L., FLEMING L. M. A mapping of oxidative enzymes in the human brain. J Neurochem. 1962 Mar-Apr;9:179–198. doi: 10.1111/j.1471-4159.1962.tb11860.x. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990 Jun;4(9):2587–2597. [PubMed] [Google Scholar]
- Groner Y., Lieman-Hurwitz J., Dafni N., Sherman L., Levanon D., Bernstein Y., Danciger E., Elroy-Stein O. Molecular structure and expression of the gene locus on chromosome 21 encoding the Cu/Zn superoxide dismutase and its relevance to Down syndrome. Ann N Y Acad Sci. 1985;450:133–156. doi: 10.1111/j.1749-6632.1985.tb21489.x. [DOI] [PubMed] [Google Scholar]
- Hensley K., Carney J. M., Mattson M. P., Aksenova M., Harris M., Wu J. F., Floyd R. A., Butterfield D. A. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M., Fujii J., Nagai Y., Sonobe M., Okamoto K., Araki H., Taniguchi N., Ueno S. A new variant Cu/Zn superoxide dismutase (Val7-->Glu) deduced from lymphocyte mRNA sequences from Japanese patients with familial amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 1994 Oct 28;204(2):572–577. doi: 10.1006/bbrc.1994.2497. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative damage in neurodegenerative disease. Lancet. 1994 Sep 17;344(8925):796–798. doi: 10.1016/s0140-6736(94)92347-7. [DOI] [PubMed] [Google Scholar]
- Kawamata J., Hasegawa H., Shimohama S., Kimura J., Tanaka S., Ueda K. Leu106-->Val (CTC-->GTC) mutation of superoxide dismutase-1 gene in patient with familial amyotrophic lateral sclerosis in Japan. Lancet. 1994 Jun 11;343(8911):1501–1501. doi: 10.1016/s0140-6736(94)92610-7. [DOI] [PubMed] [Google Scholar]
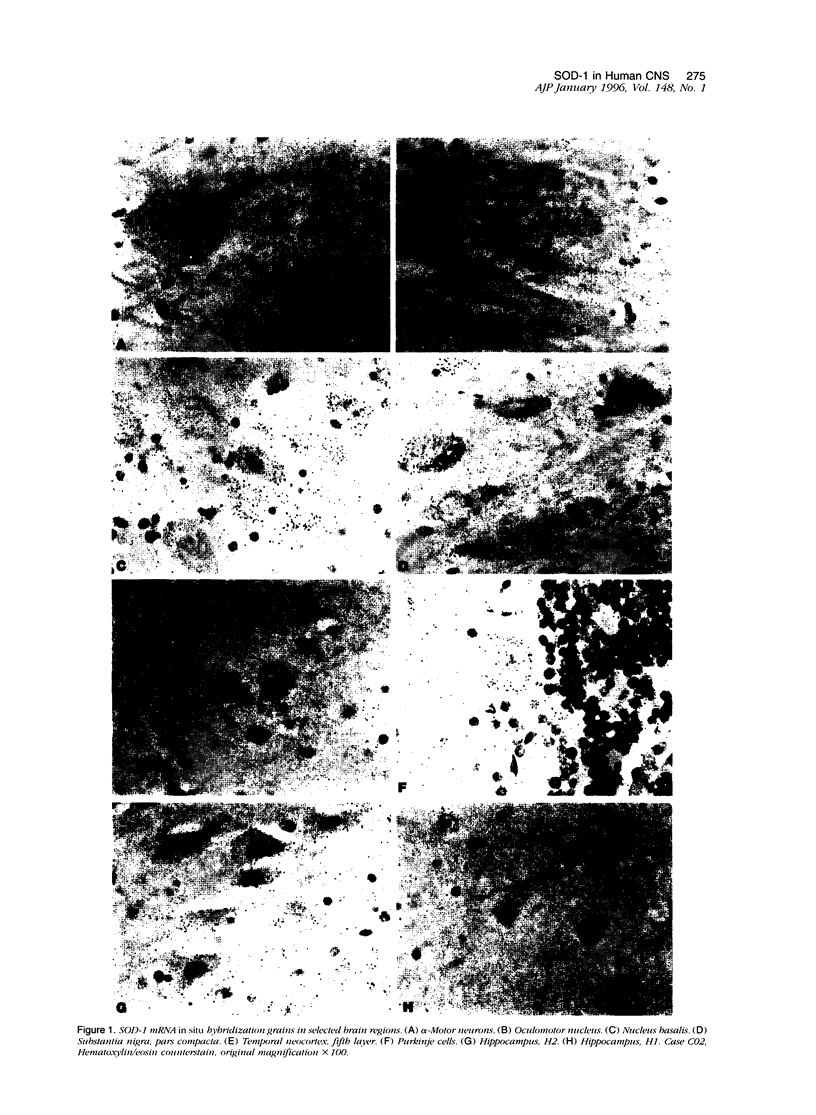
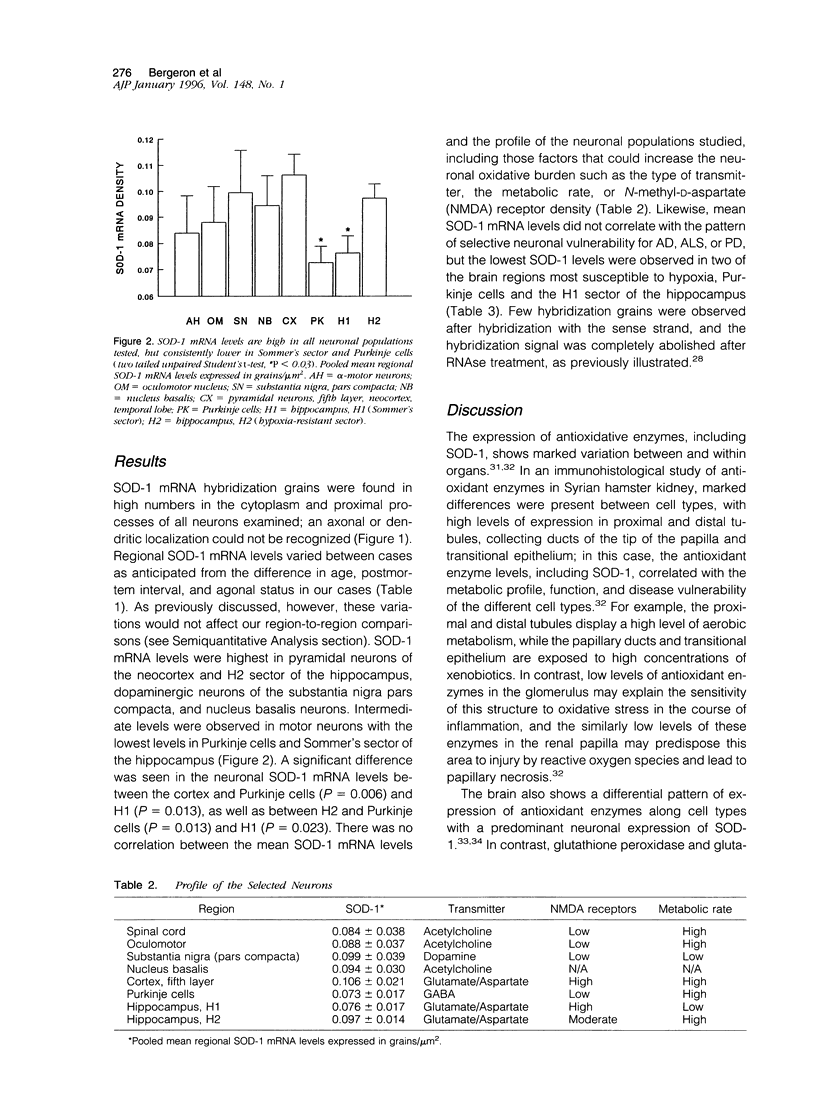
- Lanius R. A., Krieger C., Wagey R., Shaw C. A. Increased [35S]glutathione binding sites in spinal cords from patients with sporadic amyotrophic lateral sclerosis. Neurosci Lett. 1993 Nov 26;163(1):89–92. doi: 10.1016/0304-3940(93)90236-e. [DOI] [PubMed] [Google Scholar]
- Makar T. K., Nedergaard M., Preuss A., Gelbard A. S., Perumal A. S., Cooper A. J. Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem. 1994 Jan;62(1):45–53. doi: 10.1046/j.1471-4159.1994.62010045.x. [DOI] [PubMed] [Google Scholar]
- Marttila R. J., Lorentz H., Rinne U. K. Oxygen toxicity protecting enzymes in Parkinson's disease. Increase of superoxide dismutase-like activity in the substantia nigra and basal nucleus. J Neurol Sci. 1988 Sep;86(2-3):321–331. doi: 10.1016/0022-510x(88)90108-6. [DOI] [PubMed] [Google Scholar]
- Mitchell J. D., Gatt J. A., Phillips T. M., Houghton E., Rostron G., Wignall C., Whittington J., Shaw I. C. Cu/Zn superoxide dismutase free radicals, and motoneuron disease. Lancet. 1993 Oct 23;342(8878):1051–1052. doi: 10.1016/0140-6736(93)92906-a. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muse K. E., Oberley T. D., Sempf J. M., Oberley L. W. Immunolocalization of antioxidant enzymes in adult hamster kidney. Histochem J. 1994 Sep;26(9):734–753. doi: 10.1007/BF00158205. [DOI] [PubMed] [Google Scholar]
- Nakano R., Sato S., Inuzuka T., Sakimura K., Mishina M., Takahashi H., Ikuta F., Honma Y., Fujii J., Taniguchi N. A novel mutation in Cu/Zn superoxide dismutase gene in Japanese familial amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 1994 Apr 29;200(2):695–703. doi: 10.1006/bbrc.1994.1506. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Oberley L. W., Slattery A. F., Lauchner L. J., Elwell J. H. Immunohistochemical localization of antioxidant enzymes in adult Syrian hamster tissues and during kidney development. Am J Pathol. 1990 Jul;137(1):199–214. [PMC free article] [PubMed] [Google Scholar]
- Olanow C. W. An introduction to the free radical hypothesis in Parkinson's disease. Ann Neurol. 1992;32 (Suppl):S2–S9. doi: 10.1002/ana.410320703. [DOI] [PubMed] [Google Scholar]
- Pardo C. A., Xu Z., Borchelt D. R., Price D. L., Sisodia S. S., Cleveland D. W. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramatarova A., Goto J., Nanba E., Nakashima K., Takahashi K., Takagi A., Kanazawa I., Figlewicz D. A., Rouleau G. A. A two basepair deletion in the SOD 1 gene causes familial amyotrophic lateral sclerosis. Hum Mol Genet. 1994 Nov;3(11):2061–2062. [PubMed] [Google Scholar]
- Raps S. P., Lai J. C., Hertz L., Cooper A. J. Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res. 1989 Jul 31;493(2):398–401. doi: 10.1016/0006-8993(89)91178-5. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Bowling A. C., Patterson D., Usdin T. B., Sapp P., Mezey E., McKenna-Yasek D., O'Regan J., Rahmani Z., Ferrante R. J. A frequent ala 4 to val superoxide dismutase-1 mutation is associated with a rapidly progressive familial amyotrophic lateral sclerosis. Hum Mol Genet. 1994 Jun;3(6):981–987. doi: 10.1093/hmg/3.6.981. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Saggu H., Cooksey J., Dexter D., Wells F. R., Lees A., Jenner P., Marsden C. D. A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. J Neurochem. 1989 Sep;53(3):692–697. doi: 10.1111/j.1471-4159.1989.tb11759.x. [DOI] [PubMed] [Google Scholar]
- Slivka A., Mytilineou C., Cohen G. Histochemical evaluation of glutathione in brain. Brain Res. 1987 Apr 21;409(2):275–284. doi: 10.1016/0006-8993(87)90712-8. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Carney J. M., Starke-Reed P. E., Oliver C. N., Stadtman E. R., Floyd R. A., Markesbery W. R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Kutty R. K., Richey P. L., Yan S. D., Stern D., Chader G. J., Wiggert B., Petersen R. B., Perry G. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer's disease. Am J Pathol. 1994 Jul;145(1):42–47. [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Taneda S., Richey P. L., Miyata S., Yan S. D., Stern D., Sayre L. M., Monnier V. M., Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville M. J., Percy M. E., Bergeron C., Yoong L. K., Grima E. A., McLachlan D. R. Localization and quantitation of 68 kDa neurofilament and superoxide dismutase-1 mRNA in Alzheimer brains. Brain Res Mol Brain Res. 1991 Jan;9(1-2):1–8. doi: 10.1016/0169-328x(91)90123-f. [DOI] [PubMed] [Google Scholar]
- Subbarao K. V., Richardson J. S., Ang L. C. Autopsy samples of Alzheimer's cortex show increased peroxidation in vitro. J Neurochem. 1990 Jul;55(1):342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Chen X., Schmidt A. M., Brett J., Godman G., Zou Y. S., Scott C. W., Caputo C., Frappier T., Smith M. A. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Anglade P., Hirsch E. C., Javoy-Agid F., Agid Y. Distribution of manganese-dependent superoxide dismutase in the human brain. Neuroscience. 1994 Jul;61(2):317–330. doi: 10.1016/0306-4522(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Zhang P., Damier P., Hirsch E. C., Agid Y., Ceballos-Picot I., Sinet P. M., Nicole A., Laurent M., Javoy-Agid F. Preferential expression of superoxide dismutase messenger RNA in melanized neurons in human mesencephalon. Neuroscience. 1993 Jul;55(1):167–175. doi: 10.1016/0306-4522(93)90463-p. [DOI] [PubMed] [Google Scholar]