Abstract
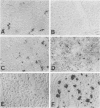
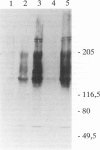
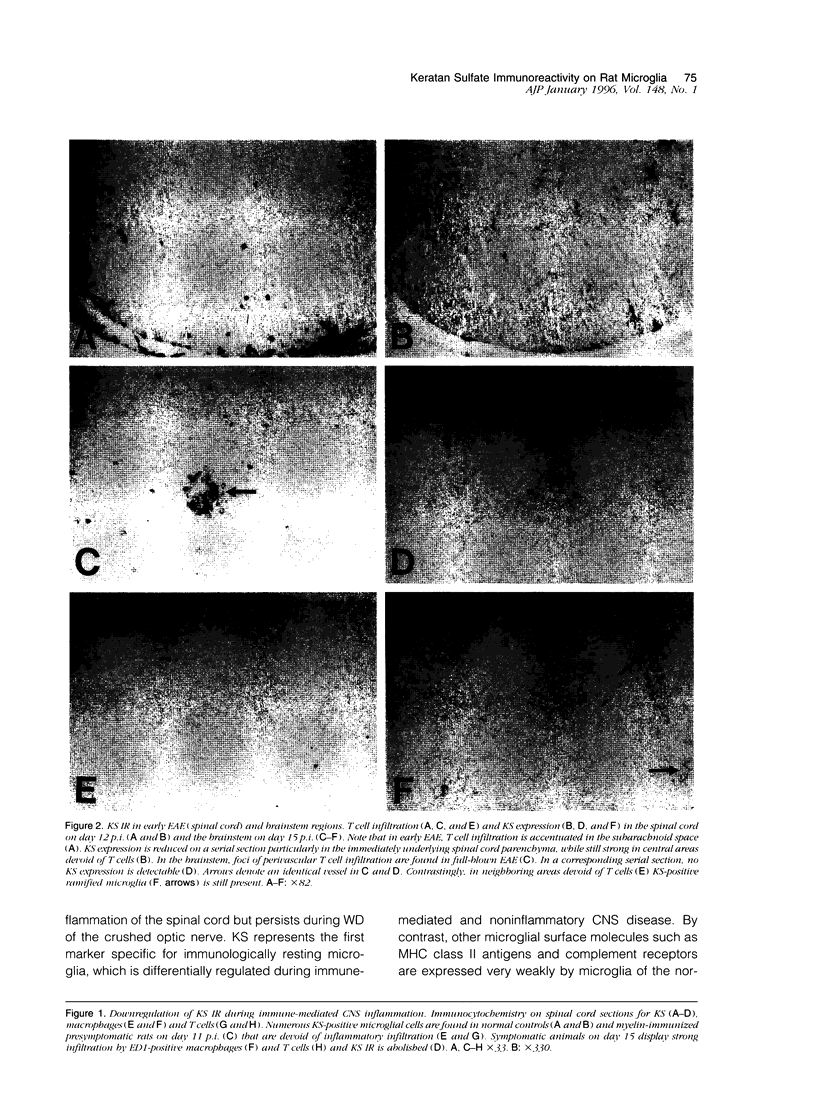
The monoclonal antibody (MAb) 5D4 against a keratan sulfate (KS) epitope of bovine cartilage proteoglycan stains ramified microglia in the rat brain. In this study we show that 5D4-positive microglia is abundant in the normal rat spinal cord and nearly absent during both the active and recovery phase of experimental autoimmune encephalomyelitis (EAE) in myelin-immunized Lewis rats. In contrast, during Wallerian degeneration of the optic nerve the density of KS-immunoreactive microglia remains constant. KS immunoreactivity is absent from both normal and transected sciatic nerves, and spinal nerve roots. On immunoblots of spinal cord extracts MAb 5D4 stains a novel type of KS proteoglycans (KSPGs) with an apparent molecular weight mainly between 140 and 200 kd, which significantly decrease in acute EAE. Our data suggest that high levels of KSPG expression correlate to a downregulated immunophenotype of resident macrophages in the nervous system. The lack of detectable KS in peripheral nerve points to a divergent differentiation of bone marrow-derived resident macrophages in the peripheral and central nervous systems and may partially account for the rapid macrophage response to axonal injury in the peripheral nervous system. Downregulation of microglial KSPG could be a prerequisite for a rapid inflammatory response in the central nervous system.
Full text
PDF


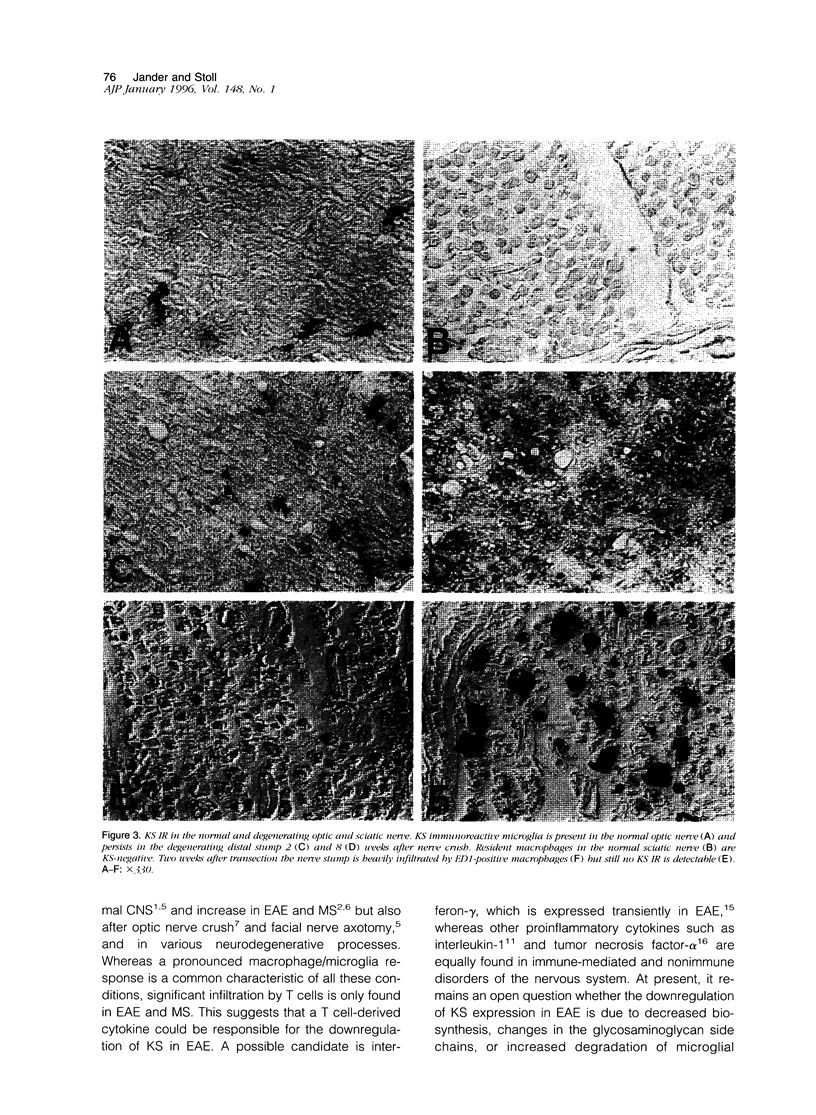
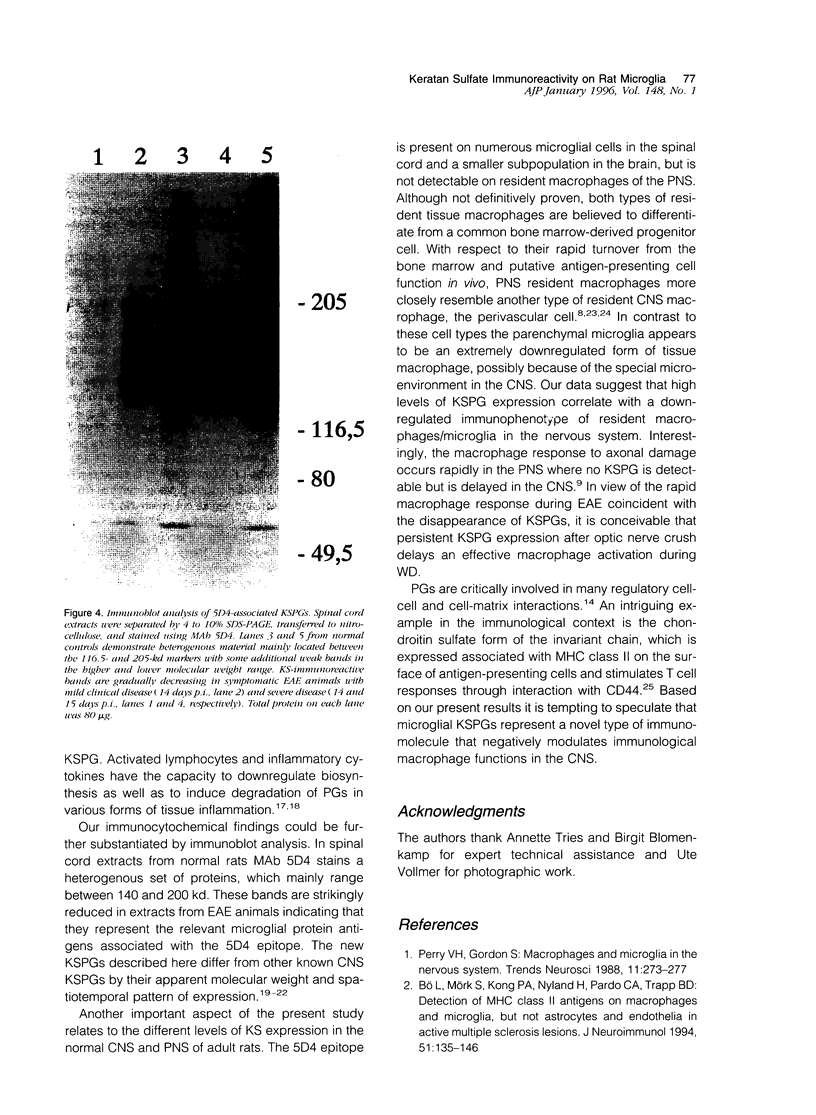

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertolotto A., Caterson B., Canavese G., Migheli A., Schiffer D. Monoclonal antibodies to keratan sulfate immunolocalize ramified microglia in paraffin and cryostat sections of rat brain. J Histochem Cytochem. 1993 Apr;41(4):481–487. doi: 10.1177/41.4.8450191. [DOI] [PubMed] [Google Scholar]
- Bö L., Mörk S., Kong P. A., Nyland H., Pardo C. A., Trapp B. D. Detection of MHC class II-antigens on macrophages and microglia, but not on astrocytes and endothelia in active multiple sclerosis lesions. J Neuroimmunol. 1994 May;51(2):135–146. doi: 10.1016/0165-5728(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983 Jul 25;258(14):8848–8854. [PubMed] [Google Scholar]
- Cole G. J., McCabe C. F. Identification of a developmentally regulated keratan sulfate proteoglycan that inhibits cell adhesion and neurite outgrowth. Neuron. 1991 Dec;7(6):1007–1018. doi: 10.1016/0896-6273(91)90345-z. [DOI] [PubMed] [Google Scholar]
- David S., Bouchard C., Tsatas O., Giftochristos N. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron. 1990 Oct;5(4):463–469. doi: 10.1016/0896-6273(90)90085-t. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Lee S. C., Mattiace L. A., Yen S. H., Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia. 1993 Jan;7(1):75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- Geisert E. E., Jr, Williams R. C., Bidanset D. J. A CNS specific proteoglycan associated with astrocytes in rat optic nerve. Brain Res. 1992 Jan 31;571(1):165–168. doi: 10.1016/0006-8993(92)90526-f. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Kreutzberg G. W. Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res. 1989 Jan;22(1):103–106. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Meyer M., Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987 Dec 17;330(6149):658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Wong W. C. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993 Jan;7(1):9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Hara N., Tanaka R., Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986 May 15;136(10):3668–3676. [PubMed] [Google Scholar]
- Naparstek Y., Cohen I. R., Fuks Z., Vlodavsky I. Activated T lymphocytes produce a matrix-degrading heparan sulphate endoglycosidase. Nature. 1984 Jul 19;310(5974):241–244. doi: 10.1038/310241a0. [DOI] [PubMed] [Google Scholar]
- Naujokas M. F., Morin M., Anderson M. S., Peterson M., Miller J. The chondroitin sulfate form of invariant chain can enhance stimulation of T cell responses through interaction with CD44. Cell. 1993 Jul 30;74(2):257–268. doi: 10.1016/0092-8674(93)90417-o. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Brown M. C., Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987 Apr 1;165(4):1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988 Jun;11(6):273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Rauch U., Gao P., Janetzko A., Flaccus A., Hilgenberg L., Tekotte H., Margolis R. K., Margolis R. U. Isolation and characterization of developmentally regulated chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of brain identified with monoclonal antibodies. J Biol Chem. 1991 Aug 5;266(22):14785–14801. [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow D. M., Lemmon V., Carrino D. A., Caplan A. I., Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990 Jul;109(1):111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Stoll G., Jung S., Jander S., van der Meide P., Hartung H. P. Tumor necrosis factor-alpha in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. J Neuroimmunol. 1993 Jun;45(1-2):175–182. doi: 10.1016/0165-5728(93)90178-2. [DOI] [PubMed] [Google Scholar]
- Stoll G., Müller S., Schmidt B., van der Meide P., Jung S., Toyka K. V., Hartung H. P. Localization of interferon-gamma and Ia-antigen in T cell line-mediated experimental autoimmune encephalomyelitis. Am J Pathol. 1993 Jun;142(6):1866–1875. [PMC free article] [PubMed] [Google Scholar]
- Stoll G., Trapp B. D., Griffin J. W. Macrophage function during Wallerian degeneration of rat optic nerve: clearance of degenerating myelin and Ia expression. J Neurosci. 1989 Jul;9(7):2327–2335. doi: 10.1523/JNEUROSCI.09-07-02327.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Vass K., Hickey W. F., Schmidt R. E., Lassmann H. Bone marrow-derived elements in the peripheral nervous system. An immunohistochemical and ultrastructural investigation in chimeric rats. Lab Invest. 1993 Sep;69(3):275–282. [PubMed] [Google Scholar]