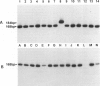
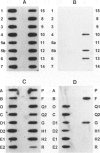
Abstract
Both Epstein-Barr virus (EBV) types A and B are found in endemic Burkitt's lymphoma (BL) occurring in equatorial Africa. We studied 17 cases of Brazilian BL previously demonstrated to be EBV-positive to determine the EBV type as well as the presence of a characteristic 30 bp deletion within the 3' end of the latent membrane protein-1 (LMP-1) gene that may be important to the pathogenesis of several EBV-associated neoplasms. All cases in which the age was known were children. We found type A EBV in 13 of 14 (93%) evaluable cases, and type B in one case. The LMP-1 deletion was found in 12 of 15 (80%) evaluable cases, including the one case of type B EBV, and a similar high prevalence (59%) of the deletion was detected in EBV-positive normal and reactive lymphoid tissues from individuals from the same geographic region. The high proportion of cases associated with type A EBV suggests that immunodeficiency is not an important factor in the pathogenesis of Brazilian BL, in contrast to endemic African BL. The presence of the LMP-1 deletion in a high prevalence in the normal population in this region is unexplained.
Full text
PDF


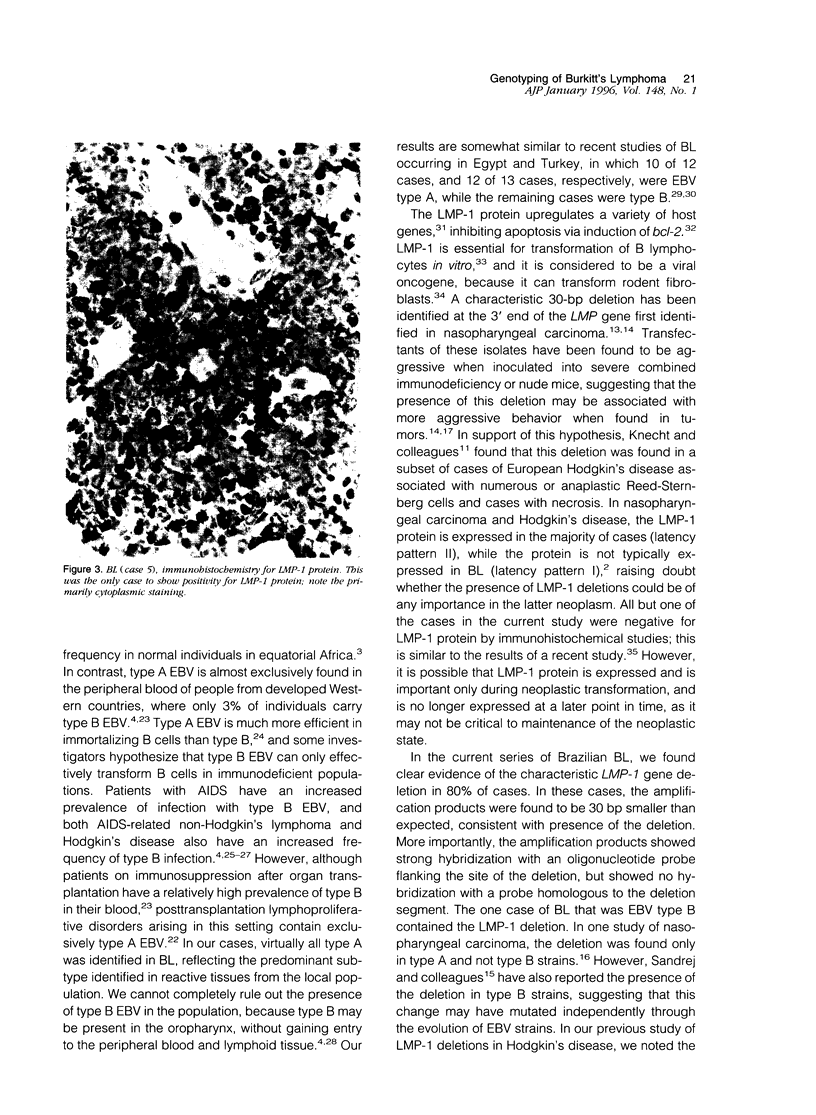


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus J. L., Ambrus C. M. Burkitt's lymphoma. J Med. 1981;12(6):385–413. [PubMed] [Google Scholar]
- Borisch B., Finke J., Hennig I., Delacrétaz F., Schneider J., Heitz P. U., Laissue J. A. Distribution and localization of Epstein-Barr virus subtypes A and B in AIDS-related lymphomas and lymphatic tissue of HIV-positive patients. J Pathol. 1992 Oct;168(2):229–236. doi: 10.1002/path.1711680212. [DOI] [PubMed] [Google Scholar]
- Boyle M. J., Sewell W. A., Sculley T. B., Apolloni A., Turner J. J., Swanson C. E., Penny R., Cooper D. A. Subtypes of Epstein-Barr virus in human immunodeficiency virus-associated non-Hodgkin lymphoma. Blood. 1991 Dec 1;78(11):3004–3011. [PubMed] [Google Scholar]
- Cavdar A. O., Yavuz G., Babacan E., Gözdasoglu S., Unal E., Ertem U., Pamir A., Yücesan S., Gökcora H., Uluoglu O. Burkitt's lymphoma in Turkish children: clinical, viral [EBV] and molecular studies. Leuk Lymphoma. 1994 Jul;14(3-4):323–330. doi: 10.3109/10428199409049685. [DOI] [PubMed] [Google Scholar]
- Chen M. L., Tsai C. N., Liang C. L., Shu C. H., Huang C. R., Sulitzeanu D., Liu S. T., Chang Y. S. Cloning and characterization of the latent membrane protein (LMP) of a specific Epstein-Barr virus variant derived from the nasopharyngeal carcinoma in the Taiwanese population. Oncogene. 1992 Nov;7(11):2131–2140. [PubMed] [Google Scholar]
- Drut R. M., Day S., Drut R., Meisner L. Demonstration of Epstein-Barr viral DNA in paraffin-embedded tissues of Burkitt's lymphoma from Argentina using the polymerase chain reaction and in situ hybridization. Pediatr Pathol. 1994 Jan-Feb;14(1):101–109. doi: 10.3109/15513819409022030. [DOI] [PubMed] [Google Scholar]
- Frank D., Cesarman E., Liu Y. F., Michler R. E., Knowles D. M. Posttransplantation lymphoproliferative disorders frequently contain type A and not type B Epstein-Barr virus. Blood. 1995 Mar 1;85(5):1396–1403. [PubMed] [Google Scholar]
- Gutiérrez M. I., Bhatia K., Barriga F., Diez B., Muriel F. S., de Andreas M. L., Epelman S., Risueño C., Magrath I. T. Molecular epidemiology of Burkitt's lymphoma from South America: differences in breakpoint location and Epstein-Barr virus association from tumors in other world regions. Blood. 1992 Jun 15;79(12):3261–3266. [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Hu L. F., Chen F., Zheng X., Ernberg I., Cao S. L., Christensson B., Klein G., Winberg G. Clonability and tumorigenicity of human epithelial cells expressing the EBV encoded membrane protein LMP1. Oncogene. 1993 Jun;8(6):1575–1583. [PubMed] [Google Scholar]
- Hu L. F., Zabarovsky E. R., Chen F., Cao S. L., Ernberg I., Klein G., Winberg G. Isolation and sequencing of the Epstein-Barr virus BNLF-1 gene (LMP1) from a Chinese nasopharyngeal carcinoma. J Gen Virol. 1991 Oct;72(Pt 10):2399–2409. doi: 10.1099/0022-1317-72-10-2399. [DOI] [PubMed] [Google Scholar]
- Hummel M., Anagnostopoulos I., Korbjuhn P., Stein H. Epstein-Barr virus in B-cell non-Hodgkin's lymphomas: unexpected infection patterns and different infection incidence in low- and high-grade types. J Pathol. 1995 Mar;175(3):263–271. doi: 10.1002/path.1711750303. [DOI] [PubMed] [Google Scholar]
- Kaye K. M., Izumi K. M., Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht H., Bachmann E., Brousset P., Sandvej K., Nadal D., Bachmann F., Odermatt B. F., Delsol G., Pallesen G. Deletions within the LMP1 oncogene of Epstein-Barr virus are clustered in Hodgkin's disease and identical to those observed in nasopharyngeal carcinoma. Blood. 1993 Nov 15;82(10):2937–2942. [PubMed] [Google Scholar]
- Knecht H., Bachmann E., Joske D. J., Sahli R., Eméry-Goodman A., Casanova J. L., Zilić M., Bachmann F., Odermatt B. F. Molecular analysis of the LMP (latent membrane protein) oncogene in Hodgkin's disease. Leukemia. 1993 Apr;7(4):580–585. [PubMed] [Google Scholar]
- Kyaw M. T., Hurren L., Evans L., Moss D. J., Cooper D. A., Benson E., Esmore D., Sculley T. B. Expression of B-type Epstein-Barr virus in HIV-infected patients and cardiac transplant recipients. AIDS Res Hum Retroviruses. 1992 Nov;8(11):1869–1874. doi: 10.1089/aid.1992.8.1869. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Lin S. C., De B. K., Chan W. P., Evatt B. L., Chan W. C. Precision of genotyping of Epstein-Barr virus by polymerase chain reaction using three gene loci (EBNA-2, EBNA-3C, and EBER): predominance of type A virus associated with Hodgkin's disease. Blood. 1993 Jun 15;81(12):3372–3381. [PubMed] [Google Scholar]
- Miller W. E., Edwards R. H., Walling D. M., Raab-Traub N. Sequence variation in the Epstein-Barr virus latent membrane protein 1. J Gen Virol. 1994 Oct;75(Pt 10):2729–2740. doi: 10.1099/0022-1317-75-10-2729. [DOI] [PubMed] [Google Scholar]
- Niedobitek G., Agathanggelou A., Rowe M., Jones E. L., Jones D. B., Turyaguma P., Oryema J., Wright D. H., Young L. S. Heterogeneous expression of Epstein-Barr virus latent proteins in endemic Burkitt's lymphoma. Blood. 1995 Jul 15;86(2):659–665. [PubMed] [Google Scholar]
- Rickinson A. B., Young L. S., Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987 May;61(5):1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Young L., Martin B., Chatman T., Kieff E., Rickinson A., Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990 Sep;64(9):4084–4092. doi: 10.1128/jvi.64.9.4084-4092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvej K., Peh S. C., Andresen B. S., Pallesen G. Identification of potential hot spots in the carboxy-terminal part of the Epstein-Barr virus (EBV) BNLF-1 gene in both malignant and benign EBV-associated diseases: high frequency of a 30-bp deletion in Malaysian and Danish peripheral T-cell lymphomas. Blood. 1994 Dec 15;84(12):4053–4060. [PubMed] [Google Scholar]
- Sculley T. B., Apolloni A., Hurren L., Moss D. J., Cooper D. A. Coinfection with A- and B-type Epstein-Barr virus in human immunodeficiency virus-positive subjects. J Infect Dis. 1990 Sep;162(3):643–648. doi: 10.1093/infdis/162.3.642. [DOI] [PubMed] [Google Scholar]
- Shibata D., Weiss L. M., Hernandez A. M., Nathwani B. N., Bernstein L., Levine A. M. Epstein-Barr virus-associated non-Hodgkin's lymphoma in patients infected with the human immunodeficiency virus. Blood. 1993 Apr 15;81(8):2102–2109. [PubMed] [Google Scholar]
- Shiramizu B., Barriga F., Neequaye J., Jafri A., Dalla-Favera R., Neri A., Guttierez M., Levine P., Magrath I. Patterns of chromosomal breakpoint locations in Burkitt's lymphoma: relevance to geography and Epstein-Barr virus association. Blood. 1991 Apr 1;77(7):1516–1526. [PubMed] [Google Scholar]
- Sixbey J. W., Shirley P., Chesney P. J., Buntin D. M., Resnick L. Detection of a second widespread strain of Epstein-Barr virus. Lancet. 1989 Sep 30;2(8666):761–765. doi: 10.1016/s0140-6736(89)90829-5. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Yao Q. Y., Rooney C. M., Sculley T. B., Moss D. J., Rupani H., Laux G., Bornkamm G. W., Rickinson A. B. New type B isolates of Epstein-Barr virus from Burkitt's lymphoma and from normal individuals in endemic areas. J Gen Virol. 1987 Nov;68(Pt 11):2853–2862. doi: 10.1099/0022-1317-68-11-2853. [DOI] [PubMed] [Google Scholar]