Abstract
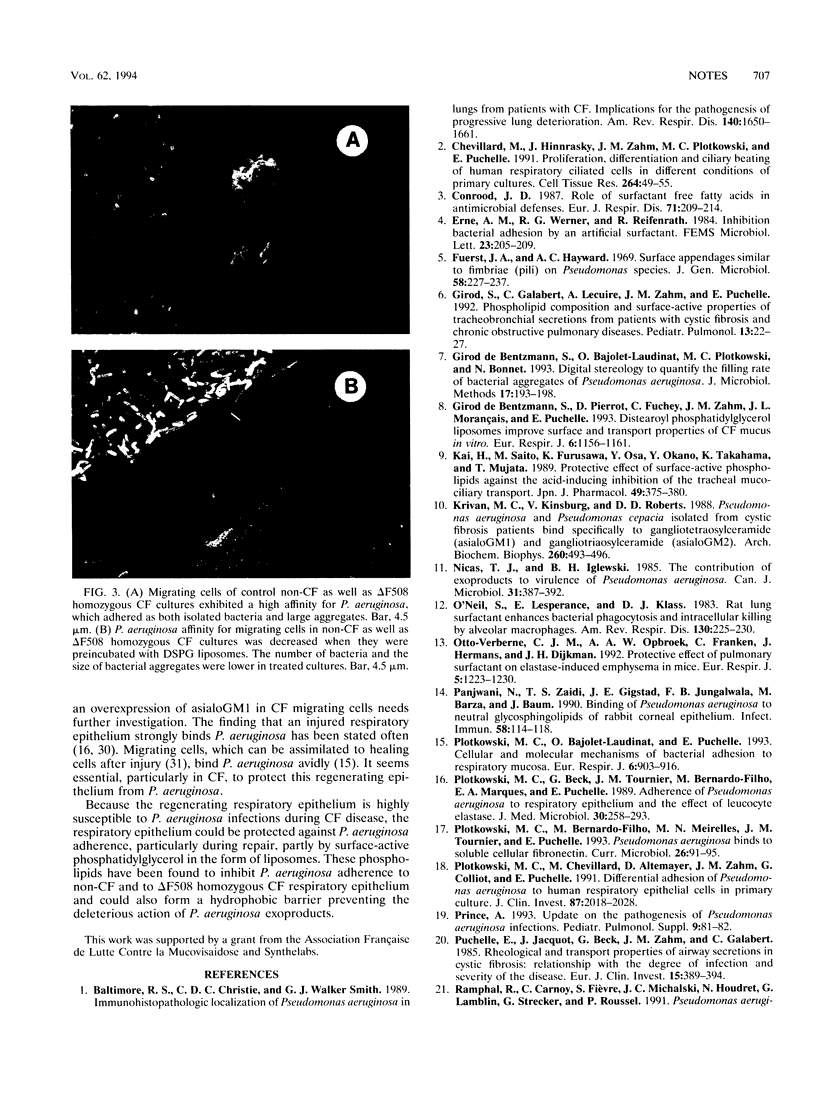
The ability of phosphatidylglycerol (DSPG) liposomes to prevent adherence of Pseudomonas aeruginosa to primary cultures of non-cystic fibrosis (CF) and delta F508 homozygous CF human respiratory epithelium was studied. The culture model was characterized by the simultaneous presence of various cellular phenotypes: well-differentiated respiratory epithelial cells, ciliated and nonciliated cells, and migrating cells which can be assimilated into a regenerating epithelium after injury. DSPG liposomes significantly decreased the binding of P. aeruginosa to migrating cells of both non-CF and delta F508 homozygous CF cultures compared with control cultures (35.5 x 10(-3) +/- 8.1 x 10(-3) bacteria per micron 2 versus 23.9 x 10(-3) +/- 2.5 x 10(-3); P < 0.01 for non-CF cultures and 88.8 x 10(-3) +/- 17.2 x 10(-3) bacteria per micron 2 versus 29.1 x 10(-3) +/- 0.6 x 10(-3), P < 0.001 for CF cultures). After treatment with DSPG liposomes, the size of P. aeruginosa aggregates bound to migrating cells in both non-CF cultures and delta F508 homozygous CF cultures was significantly decreased (14.4 +/- 3 bacteria per aggregate versus 11.9 +/- 2.5 bacteria per aggregate [P < 0.05] and 29.9 +/- 8.4 bacteria per aggregate versus 17.3 +/- 2.3 bacteria per aggregate [P < 0.01], respectively). Moreover, the control cultures were characterized by a differential P. aeruginosa adherence according to both the cellular phenotype and the mutation. The migrating cells bound more bacteria than the stationary cells of both non-CF and delta F508 homozygous CF cultures. The CF migrating cells bound significantly more bacteria than the non-CF migrating cells (88.8 x 10(-3) +/- 17.2 x 10(-3) bacteria per microns 2 versus 35.5 x 10(-3) +/- 8.1 x 10(-3) bacteria per micron 2, P < 0.001). These results suggest that DSPG liposomes are able to decrease P. aeruginosa adherence to CF and non-CF respiratory epithelium, particularly to migrating cells, which mimic a regenerating epithelium after injury. DSPG liposomes could also represent a hydrophobic barrier limiting the deleterious action of P. aeruginosa exoproducts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore R. S., Christie C. D., Smith G. J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989 Dec;140(6):1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- Chevillard M., Hinnrasky J., Zahm J. M., Plotkowski M. C., Puchelle E. Proliferation, differentiation and ciliary beating of human respiratory ciliated cells in primary culture. Cell Tissue Res. 1991 Apr;264(1):49–55. doi: 10.1007/BF00305721. [DOI] [PubMed] [Google Scholar]
- Fuerst J. A., Hayward A. C. Surface appendages similar to fimbriae (pili) on pseudomonas species. J Gen Microbiol. 1969 Oct;58(2):227–237. doi: 10.1099/00221287-58-2-227. [DOI] [PubMed] [Google Scholar]
- Girod de Bentzmann S., Pierrot D., Fuchey C., Zahm J. M., Morançais J. L., Puchelle E. Distearoyl phosphatidylglycerol liposomes improve surface and transport properties of CF mucus. Eur Respir J. 1993 Sep;6(8):1156–1161. [PubMed] [Google Scholar]
- Girod S., Galabert C., Lecuire A., Zahm J. M., Puchelle E. Phospholipid composition and surface-active properties of tracheobronchial secretions from patients with cystic fibrosis and chronic obstructive pulmonary diseases. Pediatr Pulmonol. 1992 May;13(1):22–27. doi: 10.1002/ppul.1950130107. [DOI] [PubMed] [Google Scholar]
- Kai H., Saito M., Furusawa K., Oda Y., Okano Y., Takahama K., Miyata T. Protective effect of surface-active phospholipids against the acid-inducing inhibition of the tracheal mucociliary transport. Jpn J Pharmacol. 1989 Mar;49(3):375–380. doi: 10.1254/jjp.49.375. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Iglewski B. H. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can J Microbiol. 1985 Apr;31(4):387–392. doi: 10.1139/m85-074. [DOI] [PubMed] [Google Scholar]
- O'Neill S., Lesperance E., Klass D. J. Rat lung lavage surfactant enhances bacterial phagocytosis and intracellular killing by alveolar macrophages. Am Rev Respir Dis. 1984 Aug;130(2):225–230. doi: 10.1164/arrd.1984.130.2.225. [DOI] [PubMed] [Google Scholar]
- Otto-Verberne C. J., Ten Have-Opbroek A. A., Franken C., Hermans J., Dijkman J. H. Protective effect of pulmonary surfactant on elastase-induced emphysema in mice. Eur Respir J. 1992 Nov;5(10):1223–1230. [PubMed] [Google Scholar]
- Panjwani N., Zaidi T. S., Gigstad J. E., Jungalwala F. B., Barza M., Baum J. Binding of Pseudomonas aeruginosa to neutral glycosphingolipids of rabbit corneal epithelium. Infect Immun. 1990 Jan;58(1):114–118. doi: 10.1128/iai.58.1.114-118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkowski M. C., Bajolet-Laudinat O., Puchelle E. Cellular and molecular mechanisms of bacterial adhesion to respiratory mucosa. Eur Respir J. 1993 Jun;6(6):903–916. [PubMed] [Google Scholar]
- Plotkowski M. C., Beck G., Tournier J. M., Bernardo-Filho M., Marques E. A., Puchelle E. Adherence of Pseudomonas aeruginosa to respiratory epithelium and the effect of leucocyte elastase. J Med Microbiol. 1989 Dec;30(4):285–293. doi: 10.1099/00222615-30-4-285. [DOI] [PubMed] [Google Scholar]
- Plotkowski M. C., Chevillard M., Pierrot D., Altemayer D., Zahm J. M., Colliot G., Puchelle E. Differential adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells in primary culture. J Clin Invest. 1991 Jun;87(6):2018–2028. doi: 10.1172/JCI115231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchelle E., Jacquot J., Beck G., Zahm J. M., Galabert C. Rheological and transport properties of airway secretions in cystic fibrosis--relationships with the degree of infection and severity of the disease. Eur J Clin Invest. 1985 Dec;15(6):389–394. doi: 10.1111/j.1365-2362.1985.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Houdret N., Koo L., Lamblin G., Roussel P. Differences in adhesion of Pseudomonas aeruginosa to mucin glycopeptides from sputa of patients with cystic fibrosis and chronic bronchitis. Infect Immun. 1989 Oct;57(10):3066–3071. doi: 10.1128/iai.57.10.3066-3071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Saiman L., Cacalano G., Gruenert D., Prince A. Comparison of adherence of Pseudomonas aeruginosa to respiratory epithelial cells from cystic fibrosis patients and healthy subjects. Infect Immun. 1992 Jul;60(7):2808–2814. doi: 10.1128/iai.60.7.2808-2814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L., Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Invest. 1993 Oct;92(4):1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U., Reisman J., Doig P., Irvin R. T., Forstner G., Forstner J. Binding of nonmucoid Pseudomonas aeruginosa to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992 Feb;89(2):657–665. doi: 10.1172/JCI115632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumber-Saini S. K., Habbick B. F., Oles A. M., Schaefer J. P., Komiyama K. The role of saliva in aggregation and adherence of Pseudomonas aeruginosa in patients with cystic fibrosis. J Oral Pathol Med. 1992 Aug;21(7):299–304. doi: 10.1111/j.1600-0714.1992.tb01015.x. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Yamada H. Role of mechanical injury on airway surface in the pathogenesis of Pseudomonas aeruginosa. Am Rev Respir Dis. 1991 Nov;144(5):1147–1152. doi: 10.1164/ajrccm/144.5.1147. [DOI] [PubMed] [Google Scholar]
- Zahm J. M., Chevillard M., Puchelle E. Wound repair of human surface respiratory epithelium. Am J Respir Cell Mol Biol. 1991 Sep;5(3):242–248. doi: 10.1165/ajrcmb/5.3.242. [DOI] [PubMed] [Google Scholar]






